Spira technique is a type of nipple-sparing mastectomy that allows immediate reconstruction (IBR), ideal for ptotic breasts. Although, controversy persists regarding oncological results in breast cancer. The aim is to analyze complications, cosmetic outcomes, causes of reoperation and oncological results.
MethodsRetrospective observational analysis of patients undergone surgery during 2003–2018 in our center. Study population is based on patients with breast carcinoma or undergoing prophylactic mastectomy due to high-risk, in which a skin-sparing mastectomy with a de-epithelialized derma-fat flap (modified Spira technique) and direct to implant reconstruction was performed. Short and long-term complications, sequelae, tumor recurrence and survival rates are analyzed.
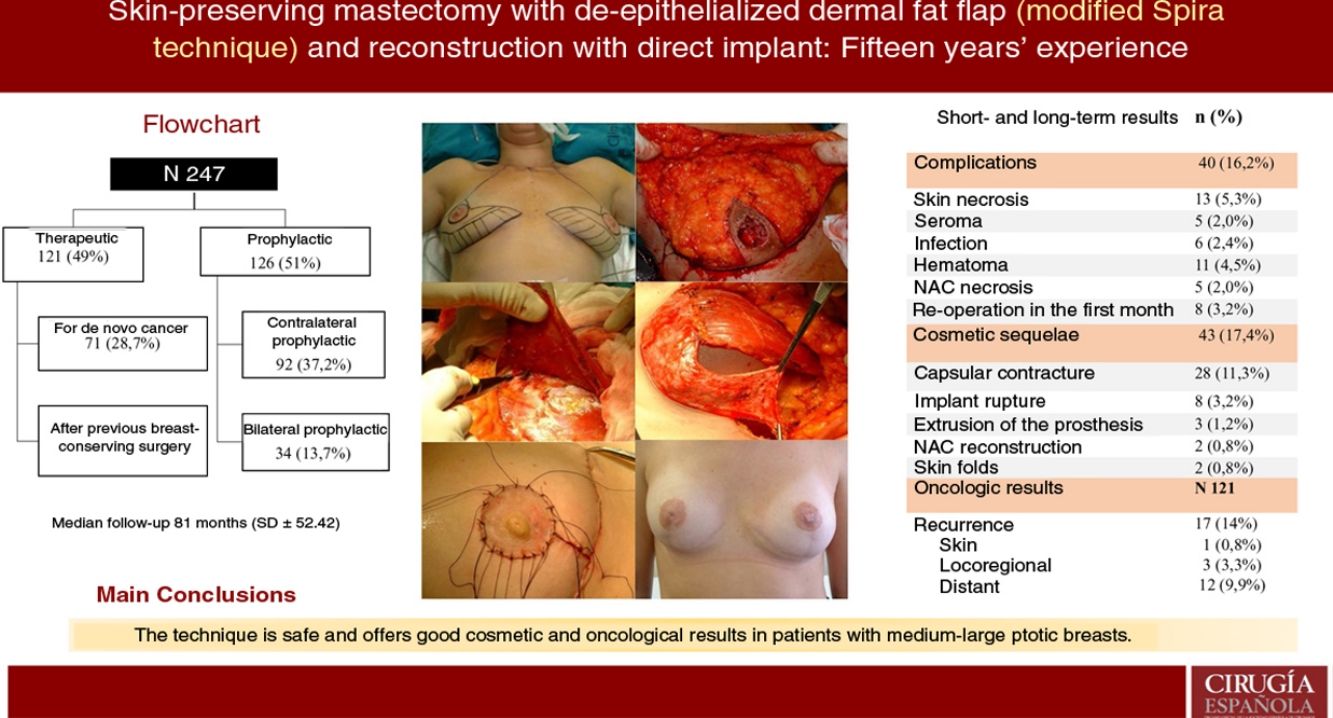
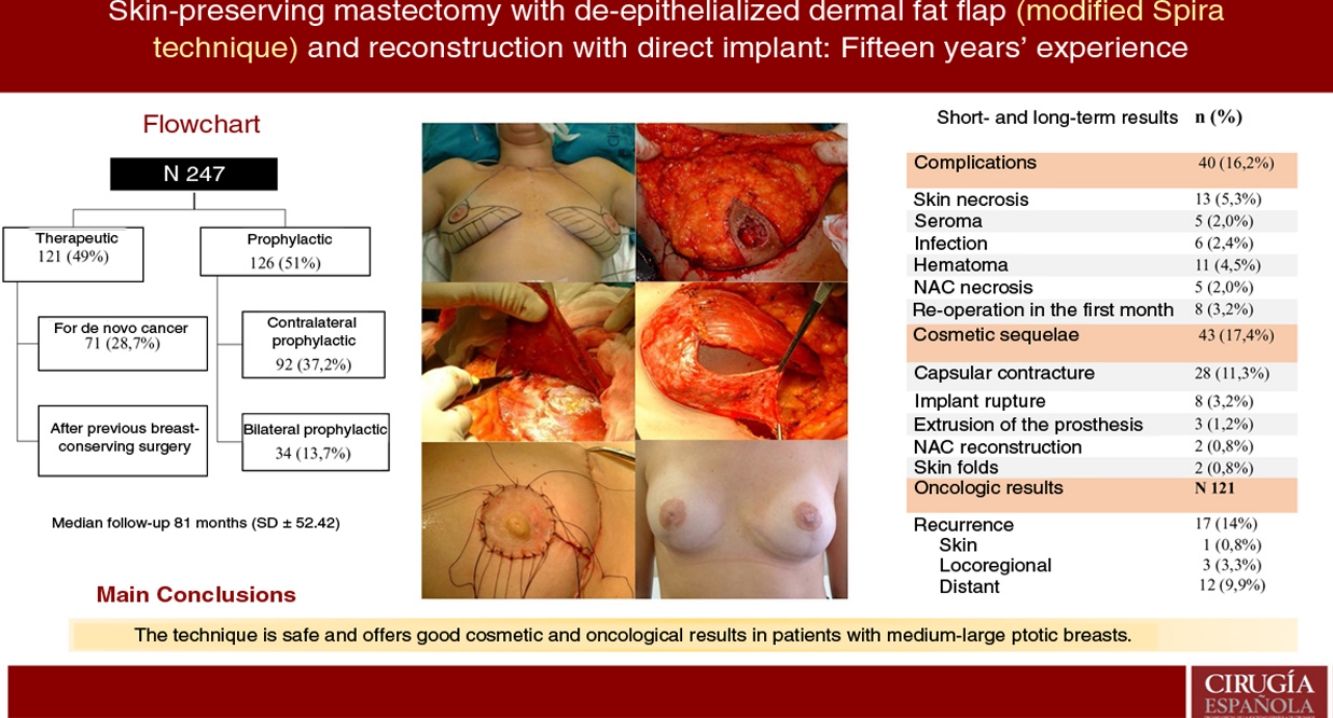
ResultsA total of 247 mastectomies with immediate reconstruction in 139 patients, 216 bilateral (87.4%) and 31 unilateral (12.5%) were performed. 121 therapeutic (49%) and 126 prophylactic (51%). Median follow-up 81 months. Complications were observed in 16.2%; skin necrosis 5.3% and 5 cases of NAC necrosis (2%). Reoperation rate due to cosmetic sequelae was 17.4% (capsular contracture was the most frequent,11.3%) and a 39.3% of these patients have received RT. Recurrence of 14% (0.8% skin, 3.3% locoregional and 9.9% metastatic), 8 patients died (6.6%). Rates of FSD and OS were 92.6% and 93.3% respectively.
ConclusionSpira mastectomy is a safe option and provides good cosmetic and oncologic results as breast cancer treatment and prophylaxis in moderate-large ptotic breasts.
La técnica de Spira es un tipo de mastectomía preservadora de piel que permite la reconstrucción inmediata (RMI), ideal en mamas ptósicas. Si bien, persiste controversia sobre resultados oncológicos en el cáncer de mama. El objetivo es analizar complicaciones, secuelas cosméticas, causas de reintervención y resultados oncológicos.
MétodosEstudio observacional retrospectivo de pacientes intervenidas durante 2003–2018 en nuestro centro. La población de estudio la constituyen pacientes con carcinoma de mama o sometidas a mastectomía profiláctica por alto riesgo, en las que se realizó una mastectomía preservadora de piel con colgajo dermograso desepitelizado (técnica de Spira modificada) y reconstrucción mediante implante directo. Se analiza la presencia de complicaciones precoces y tardías, secuelas, recidiva tumoral y supervivencia.
ResultadosSe realizaron 247 mastectomías con reconstrucción en 139 pacientes, 216 bilaterales (87,4%) y 31 unilaterales (12,5%); 121 terapéuticas (49%) y 126 profilácticas (51%). La mediana de seguimiento fue de 81 meses. Se observaron complicaciones en un 16,2%; necrosis cutánea en 5,3% y cinco casos de necrosis del CAP (2%). La tasa de reintervención por secuelas cosméticas fue del 17,4% (la más frecuente fue contractura capsular 11,3%) y, de ellas, el 39,3% recibieron RT. La tasa de recidiva fue del 14% (0,8% cutánea, 3,3% locorregional y 9,9% a distancia). Ocho pacientes fallecieron (6,6%). La SLE y SG fue del 92,6% y 93,3% a cinco años.
ConclusiónLa técnica de Spira constituye una opción segura y ofrece buenos resultados cosméticos y oncológicos como tratamiento y profilaxis de cáncer de mama en mamas ptósicas de moderado a gran tamaño.
In 1977, Spira described skin-sparing mastectomy using a lower dermal fat flap.1 Using this technique, which is classified as a Carlson type IV skin-sparing mastectomy, it is possible to remove all the glandular tissue, a small portion of skin and all the retroareolar tissue.
It is usually a technique intended for medium or large breasts with a minimal degree of ptosis. To be able to perform this technique and achieve satisfactory cosmetic results, it is essential for the nipple-areola complex (NAC) to reach at least the level of the inframammary fold.
In this technique, the NAC is removed and refined to be later grafted onto the newly created breast mound. Although Spira did not describe complications in this initial experience other than capsular contracture,1 recent studies have found general complication rates of around 20%, especially necrosis of the NAC with possible graft loss.2
The main advantage of this technique is that it allows us to perform the reconstruction in a single surgery, accommodating the definitive implant in a partially retromuscular position, covered by the dermal fat flap below. This avoids the need to use acellular dermal matrix and achieves favorable cosmetic results,3 while remaining an oncologically safe4 and efficient5 technique.
While skin-sparing mastectomy is widely validated as a therapeutic and not just prophylactic mastectomy,6 preservation of the PAC continues to generate controversy,7 mainly regarding recurrence.
The objective of this study is to analyze postoperative complications, cosmetic sequelae, causes of reoperation, and oncological results of skin-sparing mastectomy with a dermal fat flap.
MethodsPatient selectionOur study included patients who underwent skin-sparing mastectomy with a de-epithelialized dermal fat flap (modified Spira technique) and direct-to-implant reconstruction, treated consecutively over a 15-year period (2003–2018) by a single breast unit. A retrospective observational analysis was carried out.
The following clinical conditions were included:
- •
Patients with de novo breast carcinoma.
- •
Patients previously treated for breast cancer with conservative surgery who have recurrence or affected margins and opt for mastectomy instead of re-excision.
- •
Contralateral prophylactic mastectomy in patients with breast cancer (due to risk factors, patient choice, or for symmetry).
- •
Bilateral prophylactic mastectomy in patients with BRCA 1/2 mutations or high risk due to family history without demonstrated mutation.
- •
Patients with a minimal degree of ptosis (it is essential for the NAC to at least reach the level of the submammary fold).
The following cases were excluded:
- •
Inflammatory breast carcinoma
- •
Two-stage breast reconstruction
- •
Patients with distant metastases at diagnosis, since direct-to-implant reconstruction was not performed.
- •
Patients with small breasts or without ptosis (other techniques were performed).
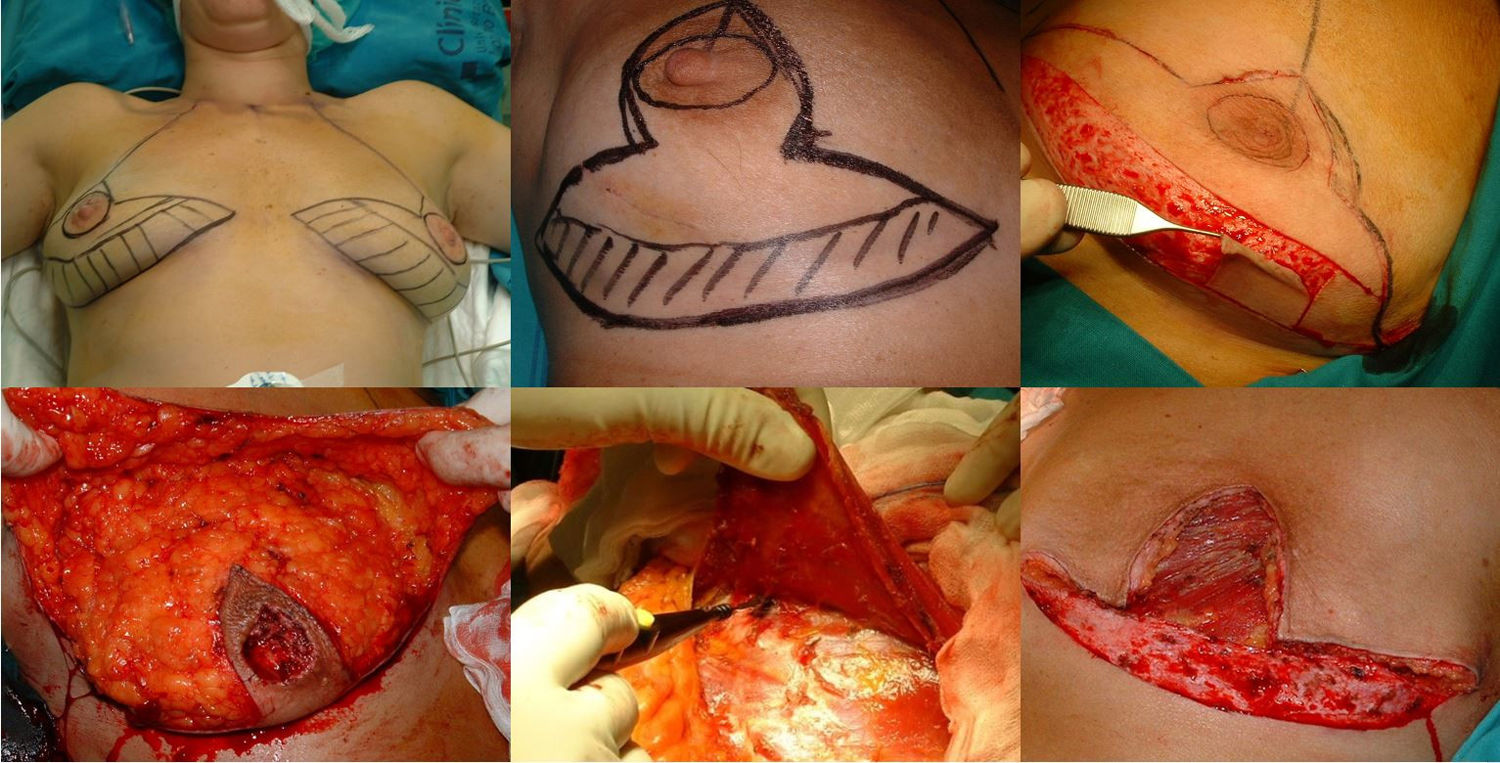
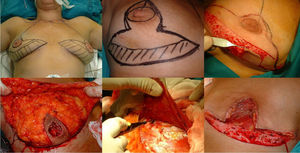
Surgical planning is carried out with the patients in the standing position. First, the skin is marked with an inverted V pattern; the submammary fold is projected on the breast midline 5 cm from the NAC, from which two vertical branches are drawn, trying to make them as closed as possible to preserve the greatest skin coverage. From there, horizontal branches are drawn towards the inframammary groove (Fig. 1).
Mastectomy phase: a) Marking the pattern; b) Inclusion of the biopsy scar within the area to be resected; c) De-epithelization of the area that will be the lower dermal fat flap; d) Excision and preservation of the NAC; e) Mastectomy releasing adhesions to the muscle; f) Result after complete excision of the gland.
The procedure is performed with the patient in a semi-sitting position. First, the NAC is released, but we do not remove it until the end of the operation (in order to decrease the ischemia time of the free graft). Then, we de-epithelialize the dermal fat flap in the lower pole of the breast. Excision of the mammary gland is completed, preserving thin flaps of skin according to the pattern that we had designed and respecting a minimum thickness of 1 cm. We remove the NAC, keeping it in gauze moistened with saline solution, and we create the dermal flap. Lastly, the axillary breast tissue is removed, and the pectoralis major muscle is released from the pectoralis minor and its attachments in the thoracic wall to later cover the implant. We also divide the pectoralis major muscle from its medial attachments at the sternum (Fig. 1).
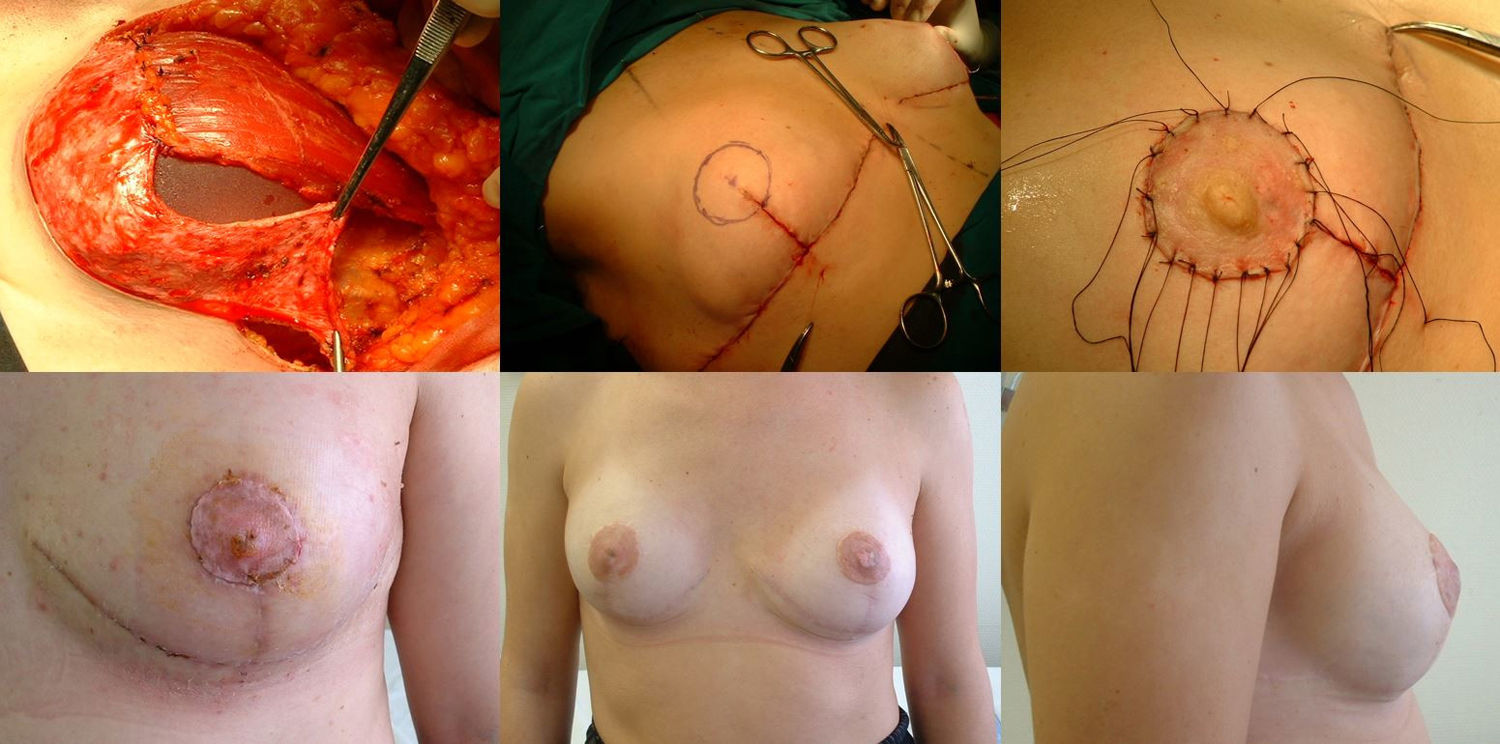
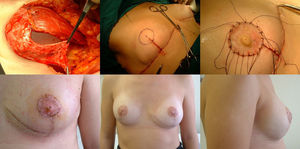
Reconstructive phaseReconstruction is completed in a single stage by placing a silicone implant with an anatomical profile, which is double covered by the de-epithelialized dermal fat flap sutured to the pectoralis major muscle. The volume of the implant depends on the volume of breast tissue extracted and the configuration of the affected and contralateral breasts, so each case is personalized. Finally, the skin is closed, following the pattern made, first the vertical branches over the projection of the submammary fold along the midline. We look for the area of maximum projection on the mound created as a new breast to mark the area where we will attach the free NAC graft. At this time, we confirm its viability by visual examination, and the absence of retroareolar tissue involvement is confirmed by intraoperative biopsy. After de-epithelializing the marked area and making small perforations in the graft, we suture the PAC using interrupted 4−0 non-absorbable monofilament stitches and place a knotted gauze pressing on the graft, which will remain in place for one week. We place two suction drains, one in the implant chamber and the other subcutaneous, which are removed when the discharge is less than 30 cc, often on an outpatient basis (Fig. 2).
Reconstruction phase: a) Placement of the implant that will be covered by the pectoralis major muscle (upper 2/3), sutured to the dermal fat flap (covering the lower 1/3); b) Marking the area for the new NAC once sutured to the skin pattern; c) Free NAC graft in its new position; d) Result after seven days; e) Result after one year; frontal view; f) Result after one year; lateral view.
The administration of chemotherapy (CT) or radiotherapy (RT) followed our hospital’s clinical practice guidelines at the time of treatment.
Study variablesThe study variables were divided into four groups:
- •
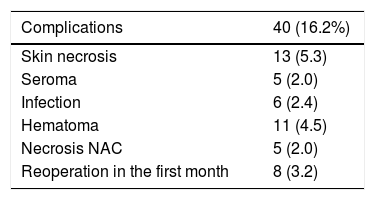
Complications: the presence of cutaneous necrosis, specifically necrosis of the NAC, which was defined as partial (possible to recover) or total (required surgical excision with secondary reconstruction); maintained seroma, infection, or hematoma.
- •
Reoperations: during the first month, they were considered ‘postoperative complications’; after the first month, they were ‘cosmetic sequelae’.
- •
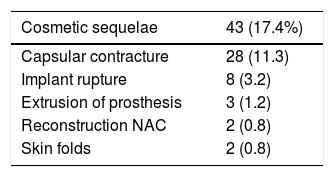
Cosmetic sequelae: include periprosthetic capsular contracture, implant rupture, prosthetic extrusion, and skin folds.
- •
Oncological results: locoregional recurrence was defined as the appearance of a new tumor in the ipsilateral chest wall (subcutaneous tissue and pectoral muscle) or recurrence in the ipsilateral axilla, supraclavicular lymph nodes, internal or infraclavicular mammary chains. Exclusively cutaneous recurrence was considered separately for the analysis. Distant recurrence (metastasis) was defined as any recurrence in all other areas not included in locoregional recurrence.
We conducted a descriptive analysis of the variables under study. For qualitative variables, relative and absolute frequencies are provided. For quantitative variables, mean and standard deviation are given. The SPSS 22.0 for Windows statistical program (SPSS Ibérica, Madrid, Spain) was used for the analysis.
This study complies with the ethical principles of the Declaration of Helsinki and was approved by the hospital ethics committee.
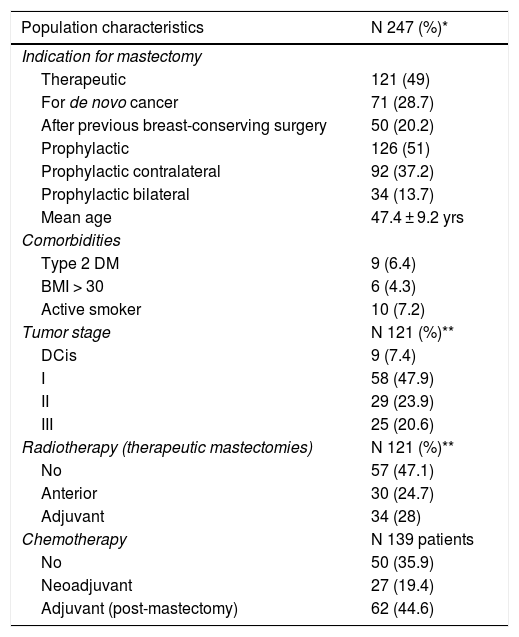
ResultsDescription of the seriesDuring the study period, 247 skin-sparing mastectomies were performed using the Spira technique, with immediate reconstruction in 139 patients. Of the total, 216 were bilateral (87.4%) and 31 unilateral (12.5%). Also, 121 therapeutic mastectomies (49%) were conducted (71 as treatment for de novo cancer [28.7%] and 50 mastectomies in breasts with previous conservative surgery [20.2%]) and 126 prophylactic mastectomies (51%) (92 prophylactic contralateral mastectomies in patients with breast cancer [37.2%] and 34 risk-reducing mastectomies in patients at risk [13.7%]). Comorbidities are shown in Table 1.
Patient characteristics.
| Population characteristics | N 247 (%)* |
|---|---|
| Indication for mastectomy | |
| Therapeutic | 121 (49) |
| For de novo cancer | 71 (28.7) |
| After previous breast-conserving surgery | 50 (20.2) |
| Prophylactic | 126 (51) |
| Prophylactic contralateral | 92 (37.2) |
| Prophylactic bilateral | 34 (13.7) |
| Mean age | 47.4 ± 9.2 yrs |
| Comorbidities | |
| Type 2 DM | 9 (6.4) |
| BMI > 30 | 6 (4.3) |
| Active smoker | 10 (7.2) |
| Tumor stage | N 121 (%)** |
| DCis | 9 (7.4) |
| I | 58 (47.9) |
| II | 29 (23.9) |
| III | 25 (20.6) |
| Radiotherapy (therapeutic mastectomies) | N 121 (%)** |
| No | 57 (47.1) |
| Anterior | 30 (24.7) |
| Adjuvant | 34 (28) |
| Chemotherapy | N 139 patients |
| No | 50 (35.9) |
| Neoadjuvant | 27 (19.4) |
| Adjuvant (post-mastectomy) | 62 (44.6) |
Post-mastectomy, 28% of patients received adjuvant RT, while 24.7% had previously received RT and 47.1% of the operated breasts were not radiated. Sixty-two patients required adjuvant CT (44.6%), 27 had received neoadjuvant CT (19.4%) and 50 did not receive chemotherapy treatment either before or after surgery (35.9%).
Adjuvant CT was administered a mean of 47.3 days after the procedure (SD 29.8) (range 13–147). In five cases (5/62; 8%), it was postponed more than the 90 days that are defined by consensus as ‘delay’, motivated in two cases by postoperative infection and in one by maintained seroma. The median follow-up was 81 months (SD 52.42).
ComplicationsThe overall complication rate was 16.2% (40/247), and skin necrosis was the most frequent (13/247 [5.3%]). There were only five cases of necrosis of the NAC after free graft (2%), and eight patients required reoperation in the short-term postoperative period (five cases due to bleeding and three due to infection, these being the patients who later presented extrusion of the prosthesis that required reoperations in the long term) (Table 2).
Cosmetic sequelaeThe rate of cosmetic sequelae was 17.4% (43/247), and grade III/IV periprosthetic capsular contracture was the most frequent cause of reoperation in the long term (28/247 [11.3%]). Out of the total number of patients who presented capsular contracture, 11 had received adjuvant RT (11/28 [39.3%]) (Table 3). Reoperation for sequelae was performed with a mean of 4.7 years (SD ± 4.96) after the intervention.
Oncological resultsOut of the 121 therapeutic mastectomies, the overall recurrence rate was 14% (17/121), with 0.8% skin recurrence, 3.3% locoregional recurrence, and 9.9% distant metastases. No recurrence was observed in the NAC.
Eight patients died (6.6%). Mean disease-free survival (DFS) was 5.08 years (SD 4.35), and mean overall survival (OS) was 5.78 years (SD 4.73). The DFS and OS rates were 92.6% and 93.3% respectively. The percentages by subgroups are shown in Table 4.
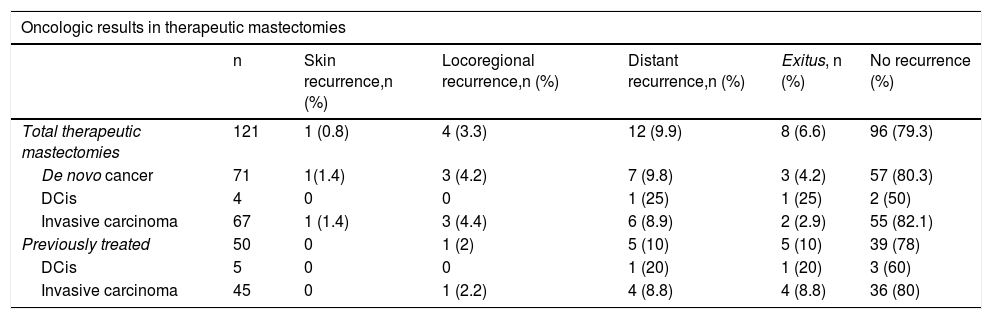
Oncologic results in therapeutic cases.
| Oncologic results in therapeutic mastectomies | ||||||
|---|---|---|---|---|---|---|
| n | Skin recurrence,n (%) | Locoregional recurrence,n (%) | Distant recurrence,n (%) | Exitus, n (%) | No recurrence (%) | |
| Total therapeutic mastectomies | 121 | 1 (0.8) | 4 (3.3) | 12 (9.9) | 8 (6.6) | 96 (79.3) |
| De novo cancer | 71 | 1(1.4) | 3 (4.2) | 7 (9.8) | 3 (4.2) | 57 (80.3) |
| DCis | 4 | 0 | 0 | 1 (25) | 1 (25) | 2 (50) |
| Invasive carcinoma | 67 | 1 (1.4) | 3 (4.4) | 6 (8.9) | 2 (2.9) | 55 (82.1) |
| Previously treated | 50 | 0 | 1 (2) | 5 (10) | 5 (10) | 39 (78) |
| DCis | 5 | 0 | 0 | 1 (20) | 1 (20) | 3 (60) |
| Invasive carcinoma | 45 | 0 | 1 (2.2) | 4 (8.8) | 4 (8.8) | 36 (80) |
Due to the increase in the detection of cancers in earlier stages, the need for skin preservation techniques has increased in recent decades in the same way as prophylactic contralateral mastectomy in patients with unilateral cancer due to patient demand.8,9 In addition, patients demand satisfactory cosmetic results and choose breast reconstruction in a single surgery, when possible.10 Therefore, we often find ourselves in the need to perform bilateral mastectomies, preserving the skin and the NAC, followed by the placement of a definitive implant, all in the same surgery. The Spira technique perfectly meets all these needs and is ideal for patients with medium or large breasts and a certain degree of ptosis.11,12
Unlike what happens with subcutaneous mastectomy in small breasts, obtaining optimal results in this type of breasts is more difficult and requires repositioning the NAC and reducing the skin coverage. The technique, which is not without risks, can compromise wound healing and the viability of the NAC, which could lead to skin necrosis with exposure of the implant, extrusion of the implant and failed reconstruction.13,14 Two mechanisms have been proposed to explain these healing/perfusion problems: first, dermal flaps that are too long; and second, flaps that are too thin, which endangers their vascularization through the subdermal plexus.15
In our experience, we found an incidence of cutaneous necrosis of 5.3%, an NAC necrosis rate of 2%, and the need for reoperation due to extrusion of the prosthesis in three patients (1.2%). These rates are lower than those reported in other series, such as the King et al. study, with 10% skin necrosis.16
Several studies have also demonstrated that the average local recurrence rate after skin-sparing mastectomies is no different whether the NAC is preserved or not (3.9% vs. 3.25%, respectively).17 Furthermore, there is evidence that combining these techniques with immediate reconstruction does not significantly delay the administration of adjuvant therapy.18 Our series supports these data, with 0.8% skin recurrence, 3.3% locoregional recurrence, and no case of recurrence in the NAC. Furthermore, we only observed a significant delay (greater than 90 days) in the administration of adjuvant chemotherapy in five cases, two due to postoperative infection and one due to a maintained seroma.
Although this study has a considerable number of cases and a long follow-up period, it presents limitations because it is a retrospective analysis of the experience from a single institution, so the results cannot be generalized.
In conclusion, skin-sparing mastectomy with de-epithelialized dermal fat flap and direct-to-implant reconstruction is a safe option that offers good cosmetic and oncological results in the treatment and prophylaxis of breast cancer for patients with moderate to large ptotic breasts.
FundingThis study has received no funding.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to thank the Breast Unit at the Hospital Clínico Universitario Lozano Blesa de Zaragoza, including surgeons, nursing staff and patients.
Please cite this article as: Allué Cabañuz M, Arribas del Amo MD, Gil Romea I, Val-Carreres Rivera MP, Sousa Domínguez R, Güemes Sánchez AT. Mastectomía preservadora de piel con colgajo dermograso desepitelizado (técnica de Spira modificada) y reconstrucción mediante implante directo. Cir Esp. 2021;99:215–221.