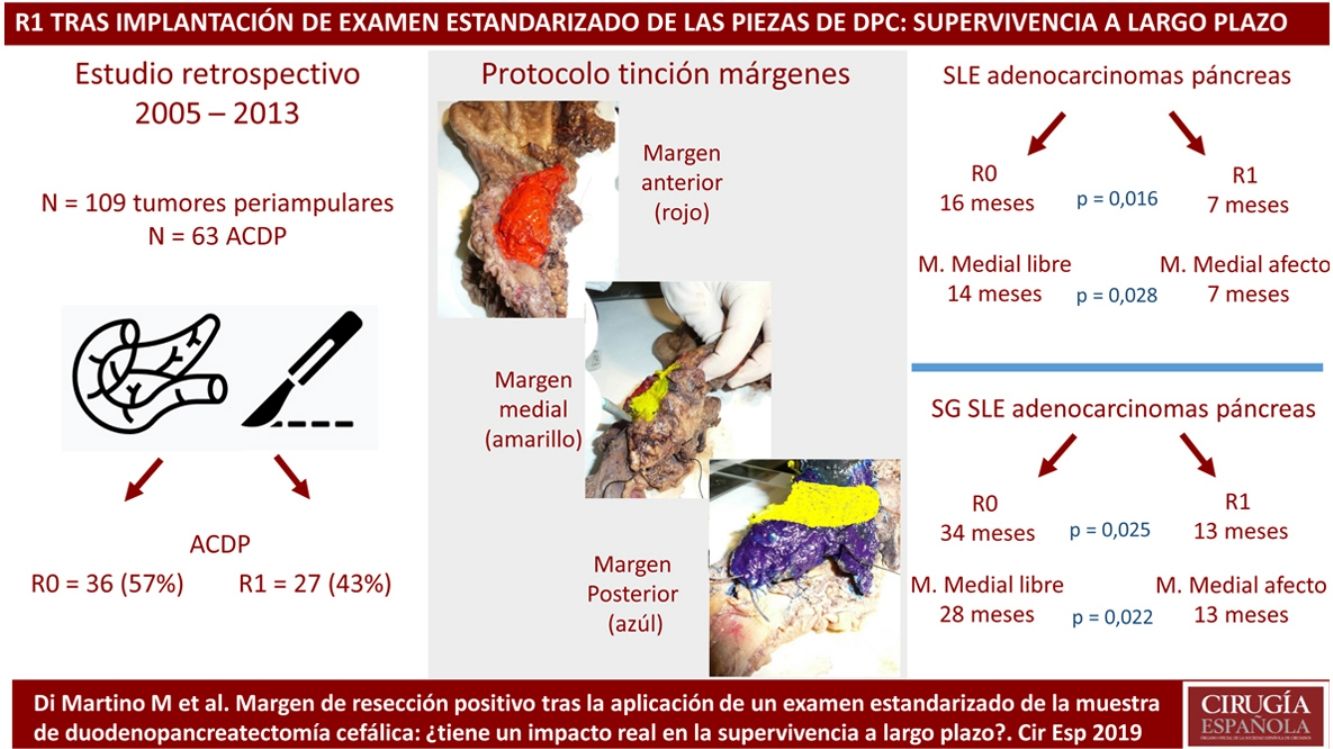
The pathological evaluation of pancreaticoduodenectomy (PD) samples and the impact of R1 resections on survival has recently been questioned. This study evaluates the introduction of a standardized pathology study protocol (PSP) and the prognosis of R1 resections after long-term follow-up.
MethodsWe reviewed data from a prospectively maintained database regarding 109 periampullary tumors treated by PD from 2005 to 2013. The results of the introduction of a PSP were analysed, and the recurrence rate (RR), disease-free survival (DFS) and overall survival (OS) of the R1 resections were evaluated for each positive margin.
ResultsThe PD specimens of periampullary tumors analyzed by PSP showed a higher rate of isolated lymph nodes (17 vs. 8; P = .003), N+ (60% vs. 31%; P < .001), microvascular invasion (67% vs. 34%; P = .001) and R1 resections (42% vs. 18%; P = .010).
Pancreatic adenocarcinomas with R1 resection in the PSP group were compared with R0, presenting higher percentages of vascular resections (P = .033), N+ (P = .029), lymphatic and perineural invasion (P = .047; P = .029), higher RR (P = .026), lower DFS (P = .016) and lower OS (P = .025). Invasion of the medial margin correlated with a worse prognosis.
ConclusionsOur series shows an increase in R1 resection after the introduction of a PSP. Infiltration of the medial margin seems to be associated with a higher RR and a decrease in DFS and OS.
La evaluación patológica de las muestras de duodenopancreatectomía cefálica (DPC) y el impacto de las resecciones R1 sobre la supervivencia ha sido recientemente cuestionado. Este estudio evalúa la introducción de un protocolo de estudio anatomopatológico estandarizado (PE) y el pronóstico de las resecciones R1 después de un seguimiento a largo plazo.
Material y métodosSe revisaron 109 tumores periampulares sometidos a DPC desde 2005 hasta 2013 a partir de una base de datos mantenida prospectivamente. Se analizaron los resultados de la introducción de un PE y se evaluaron la tasa de recurrencia (TR), supervivencia libre de enfermedad (SLE) y supervivencia global (SG) de la resección R1 para cada margen positivo.
ResultadosLas piezas de DPC de tumores periampulares analizadas mediante un PE mostraron una mayor tasa de ganglios linfáticos aislados 17 vs 8; p = 0,003, N+ 60% vs 31%; p < 0,001, invasión microvascular 67% vs 34%; p = 0,001 y resecciones R1 42% vs 18%; p = 0,010.
Se compararon los adenocarcinomas pancreáticos con resección R1 en el grupo PE con los R0, presentando mayor porcentajes de resecciones vasculares p = 0,033, N+ p = 0,029, invasión linfática y perineural p = 0,047; p = 0,029, una mayor TR p = 0,026, menor SLE p = 0,016 y menor SG p = 0,025. La infiltración del margen medial se relacionó con un peor pronóstico.
ConclusionesNuestra serie muestra un aumento en la resección R1 después de la introducción de un PE. La infiltración del margen medial parece asociarse con una mayor TR y una disminución de la SLE y SG.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in Europe,1 and surgical resection remains the only potentially curative treatment. There are striking discrepancies in the rates of R1 resections reported after pancreaticoduodenectomy (PD), ranging from 16% to 85%;2,3 in addition, R1 resection has not always correlated with a survival rate worse than R0 resection.3,4
These findings raise questions about the reliability and standardization of the pathological evaluation of PD samples. This evaluation has not been researched until recently, and there is considerable controversy over the specimen slicing technique, the definitions of resection margins (RM) and their nomenclature, with 28 terms used to define the different RM.5 The Leeds Pathology Protocol (LEEPP)2 proposed a standardized method for analyzing PD samples and defined a precise slicing technique with specific RM that have since been adopted by numerous European institutions.
The objective of this study is to compare RM involvement in PD samples before and after the implementation of a standardized protocol (SP) based on the LEEPP, analyzing the oncological prognosis after long-term follow-up according to the involvement of the different RM. First, patients whose samples were studied following an SP were compared with the non-standardized protocol group (non-SP) in order to assess differences in the R1 resection rate. Second, R1 resections of PDAC analyzed with a SP were compared with R0, evaluating the recurrence rate (RR), disease-free survival (DFS) and overall survival (OS) according to the different RM.
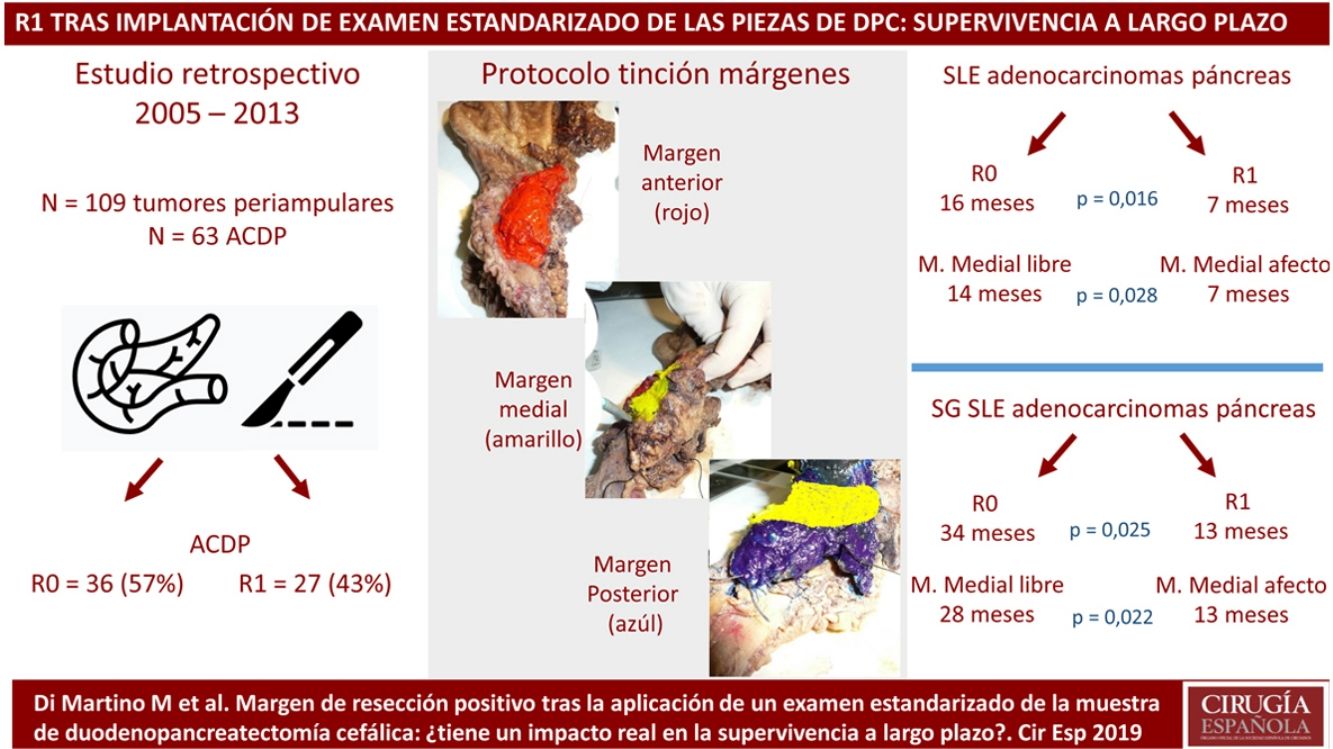
MethodsPatients and CharacteristicsA retrospective analysis was conducted of a prospectively maintained database that included all periampullary tumors treated with PD at a tertiary-level teaching hospital from 2005 to 2013. During this period, 186 patients underwent PD. After excluding benign lesions, intraductal papillary mucinous neoplasms, mucinous or serous cysts, neuroendocrine tumors and gastrointestinal stromal tumors, 109 periampullary tumors were identified, 63 of which were PDAC.
The surgical team was composed of surgeons with experience in pancreatic surgery, with no changes during the study period. The standard surgical procedure was the classic Whipple resection with Child’s loop reconstruction. Tumors that invaded the superior mesenteric vein (SMV) or portal vein (PV) were treated with venous resection.
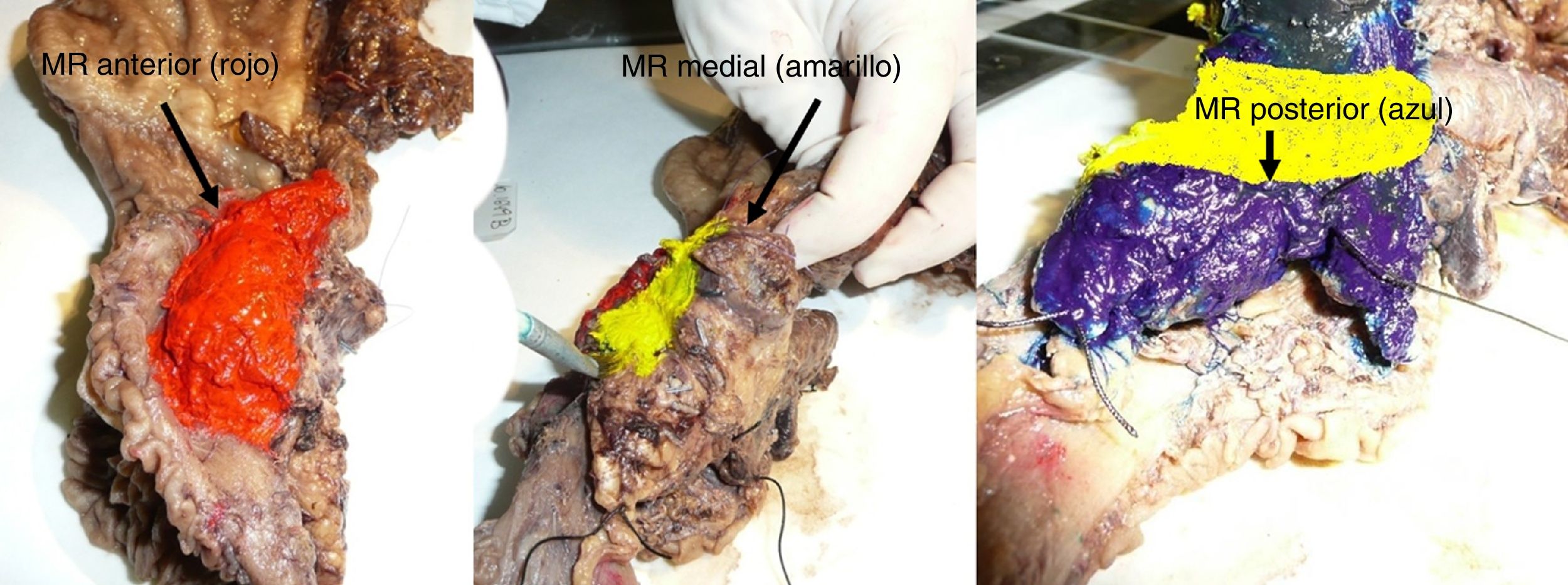
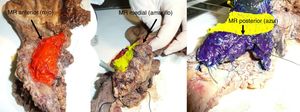
The SP was introduced in February 2009 following the LEEPP guidelines.2 It included a slicing technique on a plane perpendicular to the duodenal axis, with multicolor marking of the circumferential RM constituted by the anterior, medial, posterior and transection margins (Fig. 1). We recorded the three-dimensional size of the tumor, its relationship with the closest RM and all major anatomical structures. Samples were taken of the circumferential RM as well as the margins of the distal bile duct and stomach. The definition of R1 did not change throughout the study: R1 was defined as the presence of a tumor less than 1 mm from the RM and R0 when the distance between the tumor and the RM was greater than 1 mm.
The selection of patients who were candidates for chemotherapy or chemoradiotherapy and their regimens was based on the recommendations that were valid for each period: neoadjuvant chemoradiotherapy was used in borderline or locally advanced PDAC,6 while adjuvant chemotherapy was routinely administered in all PDAC, except when contraindicated.
For the calculation of OS and DFS, patients who died during the first 90 postoperative days were excluded. Patients were reviewed every 3 months during the first 2 postoperative years and subsequently every 6 months until the fifth year. We planned to monitor patients for a minimum period of 5 years before analyzing the cancer outcomes.
For each patient, we recorded demographic variables, existence of preoperative biliary drainage, perioperative variables and pathology study variables, including the number of lymph nodes removed. We also calculated the ratio between positive lymph nodes and the total number of resected nodes (known as the lymph node ratio) with a cut-off value of 15% (LNR15)7,8 and recorded the involvement of the different RM. Postoperative complications were classified according to the Clavien-Dindo scale.9 Pancreatic fistulae were classified according to the most recent definition of the International Study Group of Pancreatic Fistula,10 based on the measurement of amylase in the drained discharge on the third postoperative day.
Statistical AnalysisThe statistical analysis was calculated with the SPSS® v.21 program (SPSS®, Chicago, Illinois, U.S.). The qualitative variables were expressed as number and percentage. The quantitative variables were expressed as mean and standard deviation (SD) if a normal distribution was followed, or median with interquartile range when they did not follow normal distribution. Categorical variables were analyzed with Fisher’s exact test. Continuous variables were previously evaluated with the Shapiro-Wilk, Kolmogorov-Smirnoff and Levene tests (P > .05) to evaluate whether or not they followed a normal distribution and were analyzed with the Student’s t test when they followed a normal distribution, and otherwise the Mann-Whitney U was used. Survival data were analyzed using the Kaplan-Meier method and compared with the log-rank test. The differences were considered significant for P values <.05.
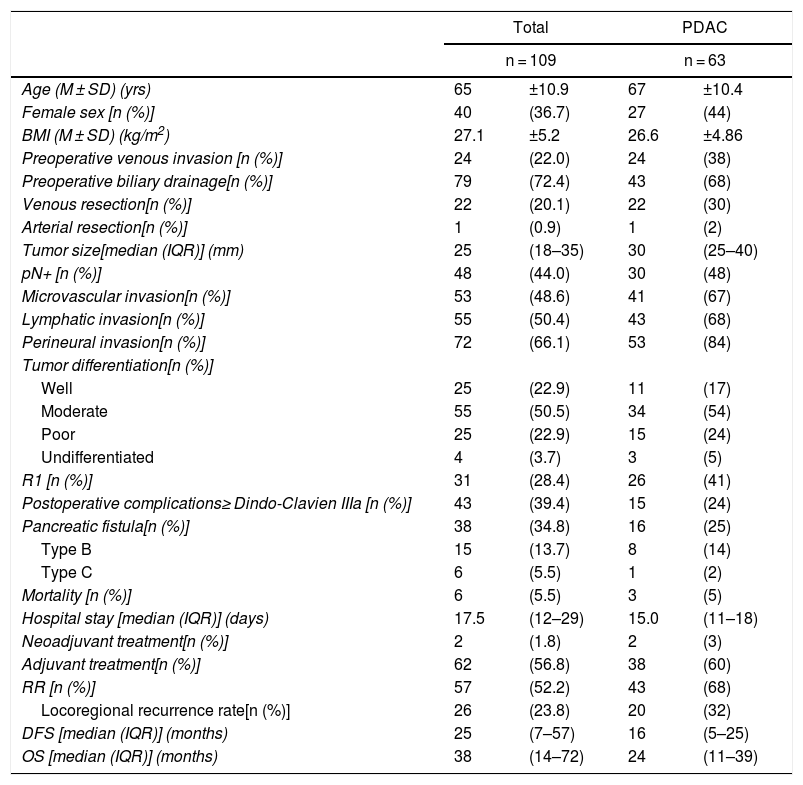
ResultsThe study included 109 patients with periampullary tumors (Table 1): 63 PDAC, 27 adenocarcinomas the ampulla of Vater, 15 cholangiocarcinomas and 4 duodenal tumors.
Demographic, Perioperative and Survival Variables.
| Total | PDAC | |||
|---|---|---|---|---|
| n = 109 | n = 63 | |||
| Age (M ± SD) (yrs) | 65 | ±10.9 | 67 | ±10.4 |
| Female sex [n (%)] | 40 | (36.7) | 27 | (44) |
| BMI (M ± SD) (kg/m2) | 27.1 | ±5.2 | 26.6 | ±4.86 |
| Preoperative venous invasion [n (%)] | 24 | (22.0) | 24 | (38) |
| Preoperative biliary drainage[n (%)] | 79 | (72.4) | 43 | (68) |
| Venous resection[n (%)] | 22 | (20.1) | 22 | (30) |
| Arterial resection[n (%)] | 1 | (0.9) | 1 | (2) |
| Tumor size[median (IQR)] (mm) | 25 | (18–35) | 30 | (25–40) |
| pN+ [n (%)] | 48 | (44.0) | 30 | (48) |
| Microvascular invasion[n (%)] | 53 | (48.6) | 41 | (67) |
| Lymphatic invasion[n (%)] | 55 | (50.4) | 43 | (68) |
| Perineural invasion[n (%)] | 72 | (66.1) | 53 | (84) |
| Tumor differentiation[n (%)] | ||||
| Well | 25 | (22.9) | 11 | (17) |
| Moderate | 55 | (50.5) | 34 | (54) |
| Poor | 25 | (22.9) | 15 | (24) |
| Undifferentiated | 4 | (3.7) | 3 | (5) |
| R1 [n (%)] | 31 | (28.4) | 26 | (41) |
| Postoperative complications≥ Dindo-Clavien IIIa [n (%)] | 43 | (39.4) | 15 | (24) |
| Pancreatic fistula[n (%)] | 38 | (34.8) | 16 | (25) |
| Type B | 15 | (13.7) | 8 | (14) |
| Type C | 6 | (5.5) | 1 | (2) |
| Mortality [n (%)] | 6 | (5.5) | 3 | (5) |
| Hospital stay [median (IQR)] (days) | 17.5 | (12–29) | 15.0 | (11–18) |
| Neoadjuvant treatment[n (%)] | 2 | (1.8) | 2 | (3) |
| Adjuvant treatment[n (%)] | 62 | (56.8) | 38 | (60) |
| RR [n (%)] | 57 | (52.2) | 43 | (68) |
| Locoregional recurrence rate[n (%)] | 26 | (23.8) | 20 | (32) |
| DFS [median (IQR)] (months) | 25 | (7–57) | 16 | (5–25) |
| OS [median (IQR)] (months) | 38 | (14–72) | 24 | (11–39) |
PDAC: pancreatic ductal adenocarcinoma; LNR15: lymph node ratio >15%; BMI: body mass index; M ± SD: mean plus–minus standard deviation; IQR: interquartile range; OS: overall survival; DFS: disease-free survival; RR: recurrence rate.
When the entire series was analyzed, mean age was 65 ± 11 years and 40 patients were women (36.7%). Venous resection was performed in 22 patients (20.1%) in whom invasion was found intraoperatively, and only one (0.9%) had arterial resection (a patient with an accessory right hepatic artery, reconstructed with a polytetrafluoroethylene prosthetic graft). Thirty-one resections (28.4%) were R1. Postoperative complications ≥ Grade IIIa according to the Clavien-Dindo classification were observed in 43 cases (39.4%). Postoperative mortality was 5.5% (6 patients), and the median hospital stay was 17.5 days (12.5–29.5 days). Adjuvant therapy was administered to 62 patients (56.8%). After the exclusion of postoperative deaths, a median DFS of 25 months (7–57 months) was observed, with an OS of 38 months (14–72 months). The data related to the PDAC subgroup are illustrated in Table 1.
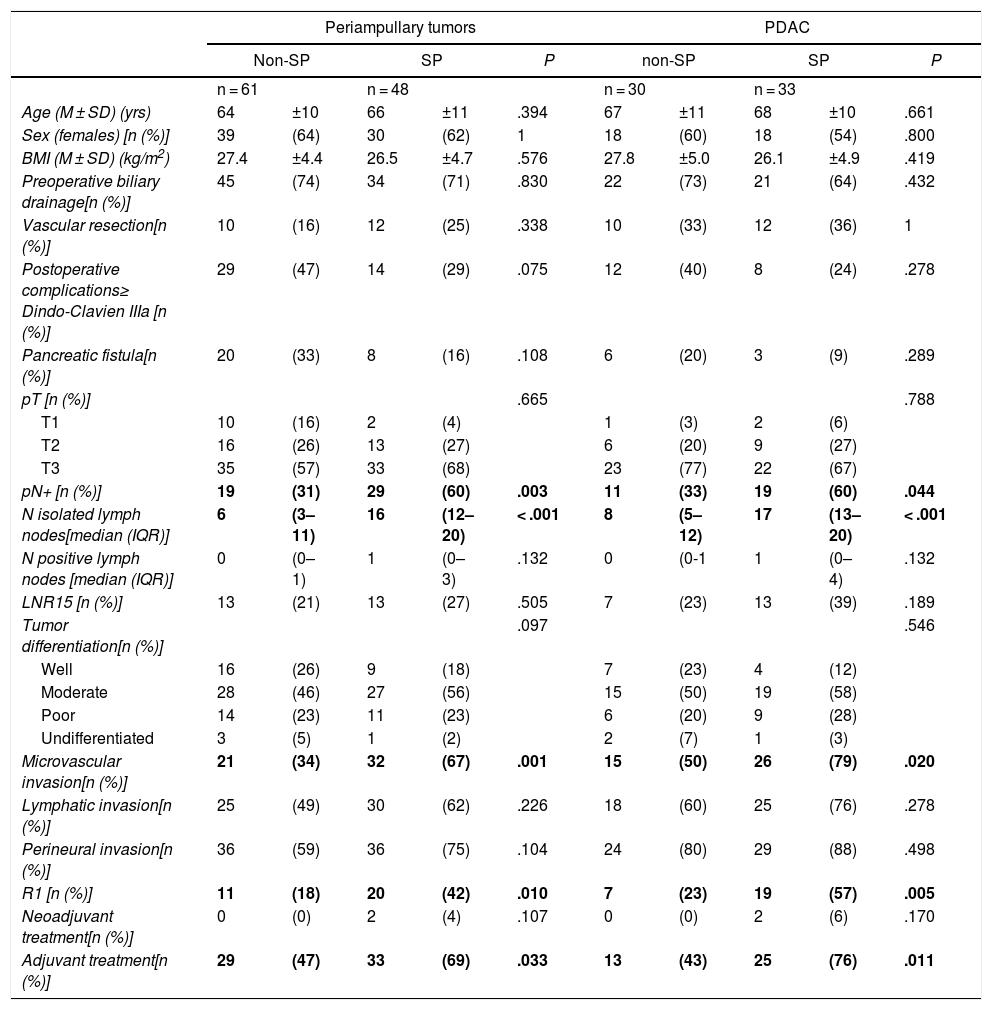
Comparison of the Findings of SP vs. Non-SP in Patients With Periampullary Tumors and in the PDAC SubgroupDue to the differences in the prognosis of the different histological types of periampullary tumors (PDAC, cholangiocarcinomas, carcinomas of the ampulla of Vater and duodenal carcinomas), after analyzing the results of the entire series as a whole, we analyzed the long-term results and the oncological prognosis of PDAC as a separate subgroup. In the periampullary tumors group, when SP was compared with non-SP, a greater number of isolated lymph nodes (17 [13–20] vs. 8 [5–17]) was observed in the SP group (P = .003), as well as a higher pN+ rate: 29 (60%) vs. 19 (31%) (P < .001); microvascular invasion: 32 (67%) vs. 21 (34%) (P = .001); and R1: 20 patients (42%) vs. 11 (18%) (P = .010). The findings of the PDAC subgroup were similar to the general group of periampullary tumors, as shown in Table 2.
Evaluation of the Standardized Study.
| Periampullary tumors | PDAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-SP | SP | P | non-SP | SP | P | |||||
| n = 61 | n = 48 | n = 30 | n = 33 | |||||||
| Age (M ± SD) (yrs) | 64 | ±10 | 66 | ±11 | .394 | 67 | ±11 | 68 | ±10 | .661 |
| Sex (females) [n (%)] | 39 | (64) | 30 | (62) | 1 | 18 | (60) | 18 | (54) | .800 |
| BMI (M ± SD) (kg/m2) | 27.4 | ±4.4 | 26.5 | ±4.7 | .576 | 27.8 | ±5.0 | 26.1 | ±4.9 | .419 |
| Preoperative biliary drainage[n (%)] | 45 | (74) | 34 | (71) | .830 | 22 | (73) | 21 | (64) | .432 |
| Vascular resection[n (%)] | 10 | (16) | 12 | (25) | .338 | 10 | (33) | 12 | (36) | 1 |
| Postoperative complications≥ Dindo-Clavien IIIa [n (%)] | 29 | (47) | 14 | (29) | .075 | 12 | (40) | 8 | (24) | .278 |
| Pancreatic fistula[n (%)] | 20 | (33) | 8 | (16) | .108 | 6 | (20) | 3 | (9) | .289 |
| pT [n (%)] | .665 | .788 | ||||||||
| T1 | 10 | (16) | 2 | (4) | 1 | (3) | 2 | (6) | ||
| T2 | 16 | (26) | 13 | (27) | 6 | (20) | 9 | (27) | ||
| T3 | 35 | (57) | 33 | (68) | 23 | (77) | 22 | (67) | ||
| pN+ [n (%)] | 19 | (31) | 29 | (60) | .003 | 11 | (33) | 19 | (60) | .044 |
| N isolated lymph nodes[median (IQR)] | 6 | (3–11) | 16 | (12–20) | < .001 | 8 | (5–12) | 17 | (13–20) | < .001 |
| N positive lymph nodes [median (IQR)] | 0 | (0–1) | 1 | (0–3) | .132 | 0 | (0-1 | 1 | (0–4) | .132 |
| LNR15 [n (%)] | 13 | (21) | 13 | (27) | .505 | 7 | (23) | 13 | (39) | .189 |
| Tumor differentiation[n (%)] | .097 | .546 | ||||||||
| Well | 16 | (26) | 9 | (18) | 7 | (23) | 4 | (12) | ||
| Moderate | 28 | (46) | 27 | (56) | 15 | (50) | 19 | (58) | ||
| Poor | 14 | (23) | 11 | (23) | 6 | (20) | 9 | (28) | ||
| Undifferentiated | 3 | (5) | 1 | (2) | 2 | (7) | 1 | (3) | ||
| Microvascular invasion[n (%)] | 21 | (34) | 32 | (67) | .001 | 15 | (50) | 26 | (79) | .020 |
| Lymphatic invasion[n (%)] | 25 | (49) | 30 | (62) | .226 | 18 | (60) | 25 | (76) | .278 |
| Perineural invasion[n (%)] | 36 | (59) | 36 | (75) | .104 | 24 | (80) | 29 | (88) | .498 |
| R1 [n (%)] | 11 | (18) | 20 | (42) | .010 | 7 | (23) | 19 | (57) | .005 |
| Neoadjuvant treatment[n (%)] | 0 | (0) | 2 | (4) | .107 | 0 | (0) | 2 | (6) | .170 |
| Adjuvant treatment[n (%)] | 29 | (47) | 33 | (69) | .033 | 13 | (43) | 25 | (76) | .011 |
PDAC: pancreatic ductal adenocarcinoma; LNR15: lymph node ratio >15%; BMI: body mass index; M ± SD: mean plus–minus standard deviation; IQR: interquartile range; SP: standardized protocol; non-SP: non-standardized protocol.
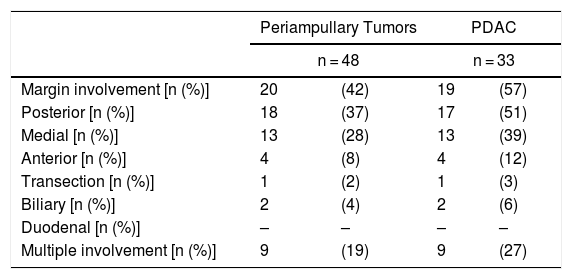
Regarding the rate of involvement of the RM, the vast majority of R1 resections were constituted by PDAC, and 17 (51%) presented invasions of the posterior RM, 13 (39%) of the medial RM and 4 (12%) of the anterior RM (Table 3). One patient had involvement of the pancreatic transection margin in the study after inclusion in paraffin, which had been considered free of tumor infiltration in the intraoperative study. Nine patients (27%) had involvement of more than one RM.
R1 Related to the Different Margins After Evaluation With SP.
| Periampullary Tumors | PDAC | |||
|---|---|---|---|---|
| n = 48 | n = 33 | |||
| Margin involvement [n (%)] | 20 | (42) | 19 | (57) |
| Posterior [n (%)] | 18 | (37) | 17 | (51) |
| Medial [n (%)] | 13 | (28) | 13 | (39) |
| Anterior [n (%)] | 4 | (8) | 4 | (12) |
| Transection [n (%)] | 1 | (2) | 1 | (3) |
| Biliary [n (%)] | 2 | (4) | 2 | (6) |
| Duodenal [n (%)] | – | – | – | – |
| Multiple involvement [n (%)] | 9 | (19) | 9 | (27) |
PDAC: pancreatic ductal adenocarcinoma; SP: standardized protocol.
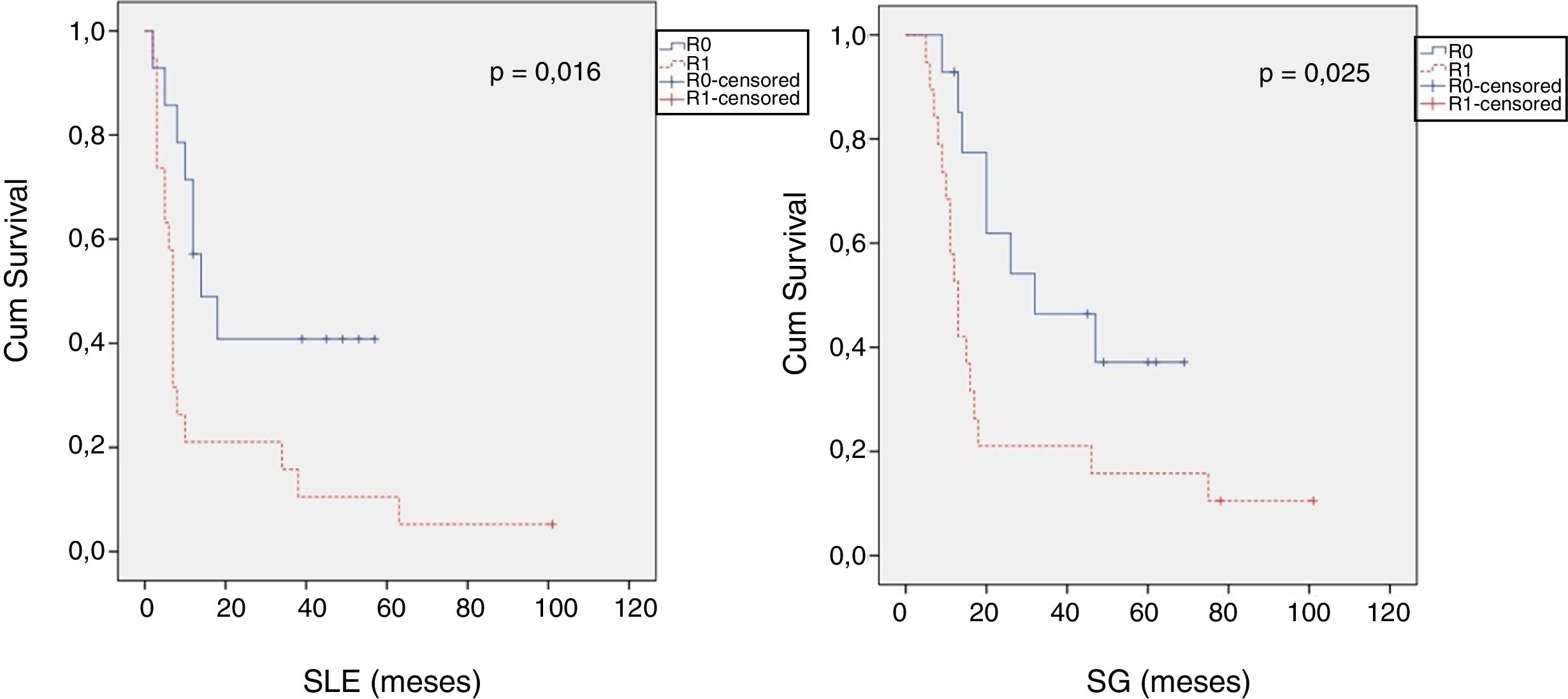
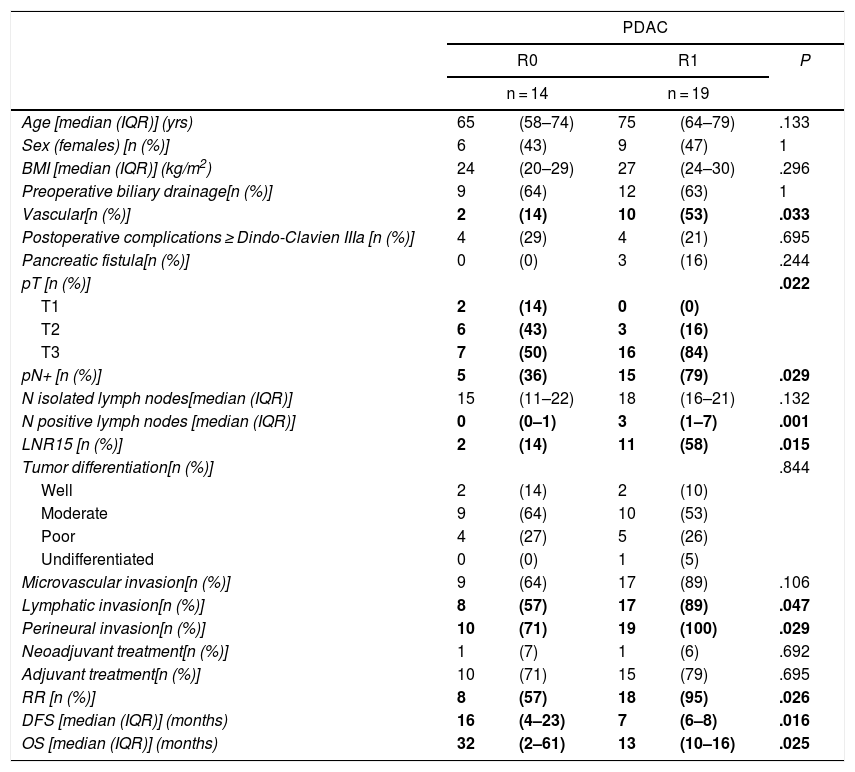
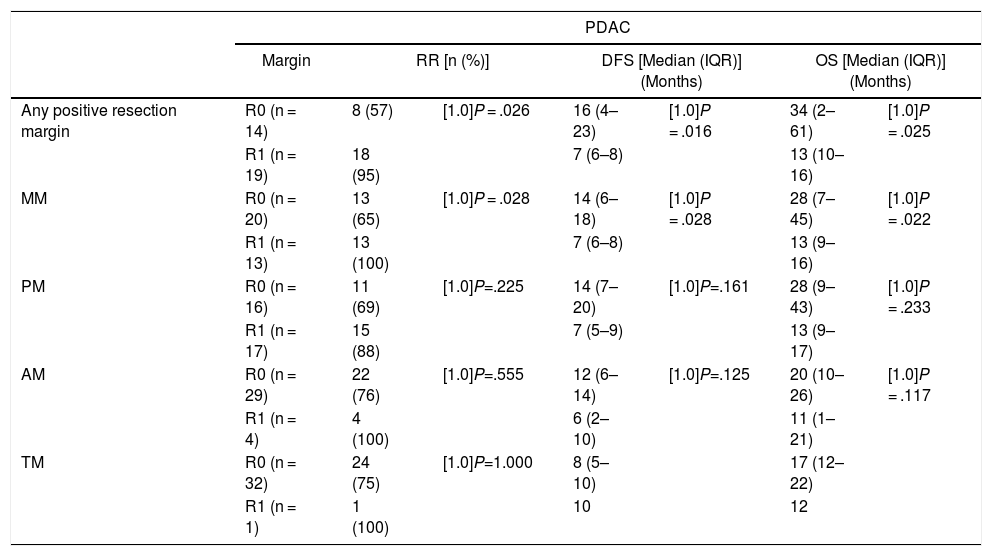
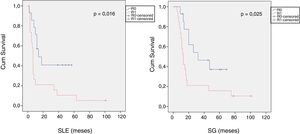
When we compared the data for R1 and R0 resections in PDAC analyzed according to an SP, the former presented a higher percentage of vascular resections (P = .033), a more advanced T stage (P = .022), higher rates of N+ (P = .029), isolated positive lymph nodes (P = .001), LNR15 (P = .015), lymphatic invasion (P = .047) and perineural invasion (P = .029) (Table 4). In addition, the R1 resection presented a higher RR (P = .026) and a lower DFS (P = .016) and OS (P = .025) (Table 4 and Fig. 2).
Comparison of R0 and R1 After the Evaluation With SP.
| PDAC | |||||
|---|---|---|---|---|---|
| R0 | R1 | P | |||
| n = 14 | n = 19 | ||||
| Age [median (IQR)] (yrs) | 65 | (58–74) | 75 | (64–79) | .133 |
| Sex (females) [n (%)] | 6 | (43) | 9 | (47) | 1 |
| BMI [median (IQR)] (kg/m2) | 24 | (20–29) | 27 | (24–30) | .296 |
| Preoperative biliary drainage[n (%)] | 9 | (64) | 12 | (63) | 1 |
| Vascular[n (%)] | 2 | (14) | 10 | (53) | .033 |
| Postoperative complications ≥ Dindo-Clavien IIIa [n (%)] | 4 | (29) | 4 | (21) | .695 |
| Pancreatic fistula[n (%)] | 0 | (0) | 3 | (16) | .244 |
| pT [n (%)] | .022 | ||||
| T1 | 2 | (14) | 0 | (0) | |
| T2 | 6 | (43) | 3 | (16) | |
| T3 | 7 | (50) | 16 | (84) | |
| pN+ [n (%)] | 5 | (36) | 15 | (79) | .029 |
| N isolated lymph nodes[median (IQR)] | 15 | (11–22) | 18 | (16–21) | .132 |
| N positive lymph nodes [median (IQR)] | 0 | (0–1) | 3 | (1–7) | .001 |
| LNR15 [n (%)] | 2 | (14) | 11 | (58) | .015 |
| Tumor differentiation[n (%)] | .844 | ||||
| Well | 2 | (14) | 2 | (10) | |
| Moderate | 9 | (64) | 10 | (53) | |
| Poor | 4 | (27) | 5 | (26) | |
| Undifferentiated | 0 | (0) | 1 | (5) | |
| Microvascular invasion[n (%)] | 9 | (64) | 17 | (89) | .106 |
| Lymphatic invasion[n (%)] | 8 | (57) | 17 | (89) | .047 |
| Perineural invasion[n (%)] | 10 | (71) | 19 | (100) | .029 |
| Neoadjuvant treatment[n (%)] | 1 | (7) | 1 | (6) | .692 |
| Adjuvant treatment[n (%)] | 10 | (71) | 15 | (79) | .695 |
| RR [n (%)] | 8 | (57) | 18 | (95) | .026 |
| DFS [median (IQR)] (months) | 16 | (4–23) | 7 | (6–8) | .016 |
| OS [median (IQR)] (months) | 32 | (2–61) | 13 | (10–16) | .025 |
PDAC: pancreatic ductal adenocarcinoma; LNR15: lymph node ratio >15%; BMI: body mass index; M ± SD: mean plus–minus standard deviation; SP: standardized protocol; IQR: interquartile range; OS: overall survival; DFS: disease-free survival; RR: recurrence rate.
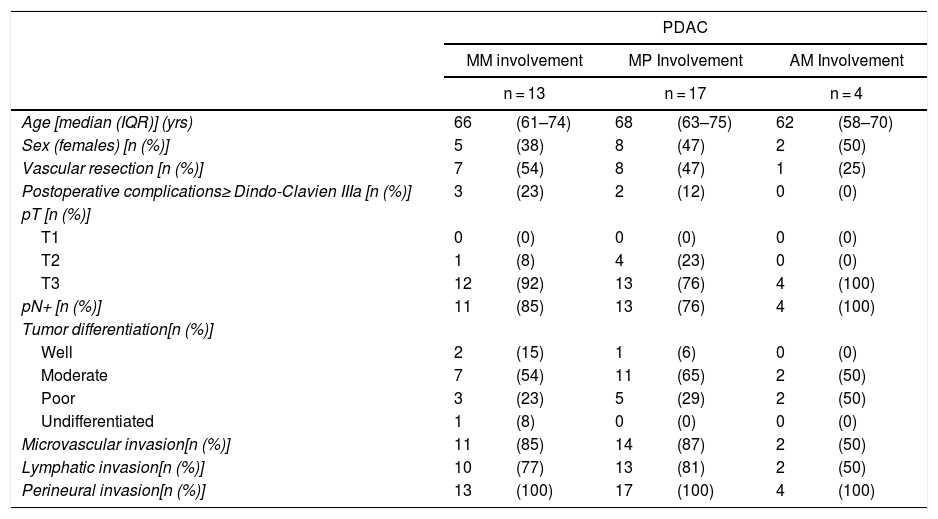
Table 5 illustrates the demographic and perioperative variables related with the the different types of RM involvement. When we analyzed the impact of the involvement of each RM on the long-term oncological prognosis, we found that only the positivity of the medial RM correlated with a higher RR (P = .028) and a lower DFS (P = .028) and OS (P = .022), while the positivity of the anterior, posterior and transection RM showed no significant differences in the long-term oncological prognosis (Table 6).
Demographic and Perioperative Variables Related With the Involvement of the Different Resection Margins.
| PDAC | ||||||
|---|---|---|---|---|---|---|
| MM involvement | MP Involvement | AM Involvement | ||||
| n = 13 | n = 17 | n = 4 | ||||
| Age [median (IQR)] (yrs) | 66 | (61–74) | 68 | (63–75) | 62 | (58–70) |
| Sex (females) [n (%)] | 5 | (38) | 8 | (47) | 2 | (50) |
| Vascular resection [n (%)] | 7 | (54) | 8 | (47) | 1 | (25) |
| Postoperative complications≥ Dindo-Clavien IIIa [n (%)] | 3 | (23) | 2 | (12) | 0 | (0) |
| pT [n (%)] | ||||||
| T1 | 0 | (0) | 0 | (0) | 0 | (0) |
| T2 | 1 | (8) | 4 | (23) | 0 | (0) |
| T3 | 12 | (92) | 13 | (76) | 4 | (100) |
| pN+ [n (%)] | 11 | (85) | 13 | (76) | 4 | (100) |
| Tumor differentiation[n (%)] | ||||||
| Well | 2 | (15) | 1 | (6) | 0 | (0) |
| Moderate | 7 | (54) | 11 | (65) | 2 | (50) |
| Poor | 3 | (23) | 5 | (29) | 2 | (50) |
| Undifferentiated | 1 | (8) | 0 | (0) | 0 | (0) |
| Microvascular invasion[n (%)] | 11 | (85) | 14 | (87) | 2 | (50) |
| Lymphatic invasion[n (%)] | 10 | (77) | 13 | (81) | 2 | (50) |
| Perineural invasion[n (%)] | 13 | (100) | 17 | (100) | 4 | (100) |
PDAC: pancreatic ductal adenocarcinoma; M ± SD: mean plus–minus standard deviation; AM: anterior margin; MM: medial margin; PM: posterior margin; TM: transection margin; IQR: interquartile range.
Comparison of RR, DFS, OS Associated With Different Resection Margins.
| PDAC | |||||||
|---|---|---|---|---|---|---|---|
| Margin | RR [n (%)] | DFS [Median (IQR)] (Months) | OS [Median (IQR)] (Months) | ||||
| Any positive resection margin | R0 (n = 14) | 8 (57) | [1.0]P = .026 | 16 (4–23) | [1.0]P = .016 | 34 (2–61) | [1.0]P = .025 |
| R1 (n = 19) | 18 (95) | 7 (6–8) | 13 (10–16) | ||||
| MM | R0 (n = 20) | 13 (65) | [1.0]P = .028 | 14 (6–18) | [1.0]P = .028 | 28 (7–45) | [1.0]P = .022 |
| R1 (n = 13) | 13 (100) | 7 (6–8) | 13 (9–16) | ||||
| PM | R0 (n = 16) | 11 (69) | [1.0]P=.225 | 14 (7–20) | [1.0]P=.161 | 28 (9–43) | [1.0]P = .233 |
| R1 (n = 17) | 15 (88) | 7 (5–9) | 13 (9–17) | ||||
| AM | R0 (n = 29) | 22 (76) | [1.0]P=.555 | 12 (6–14) | [1.0]P=.125 | 20 (10–26) | [1.0]P = .117 |
| R1 (n = 4) | 4 (100) | 6 (2–10) | 11 (1–21) | ||||
| TM | R0 (n = 32) | 24 (75) | [1.0]P=1.000 | 8 (5–10) | 17 (12–22) | ||
| R1 (n = 1) | 1 (100) | 10 | 12 | ||||
PDAC: pancreatic ductal adenocarcinoma; AM: anterior margin; MM: medial margin; PM: posterior margin; TM: transection margin; OS: overall survival; DFS: disease-free survival; RR: recurrence rate; IQR: interquartile range.
Complete resection of a tumor with an R0 margin has classically been the cornerstone of cancer surgery. However, the wide variability in the rate of R1 resections after DPC3,11–14 and its inconstant relationship with the long-term oncological prognosis opened the debate about the lack of standardization of the pathological analysis.
In 2006, Verbeke et al.2 highlighted the difficulties related to the slicing of pancreatic specimens as well as the identification and classification of the different RM. This group proposed a new standardized pathological study protocol known as LEEPP, which was subsequently adopted by the Royal College of Pathologists. In their studies,15,16 they showed that after the introduction of an SP, the rate of R1 resections increased dramatically from 53% to 85% (P = .009). Similar results were confirmed by Esposito et al.,17 who described an increase in the percentage of R1 resections from 14% to 76% (P < .001), in the French multicenter study led by Delpero et al.,18 which confirmed the increase in the percentage of R1 resection to 61% after the introduction of an SP; this was also observed by other authors.19–25 Our findings support these data, showing an increase in R1 resections with the use of an SP for the study of PD specimens both in the case of PDAC and in periampullary tumors as a whole. The impact of these results should not be underestimated: standardized analysis of surgical specimens after PDAC resection is essential for comparison between different study groups in order to rigorously evaluate the oncological prognosis of these patients as well as the best perioperative chemoradiotherapy regimens.
A recent meta-analysis26 reviewed 19 series that analyzed PD samples with an SP, confirming that medial and posterior RM were affected in approximately 50% of the samples. Our series corroborates these results, highlighting a 51% involvement of the posterior margins and a 39.4% involvement of the medial margins in patients treated surgically for PDAC.
In addition, the introduction of an SP has been shown to achieve a more detailed analysis of surgical specimens. Slidell et al.27 reported that patients with pN0 but less than 12 lymph nodes studied had a worse prognosis than pN0 tumors with a higher number of resected lymph nodes. This is probably due to understaging of the disease.28,29 Our data showed a significant difference in the number of lymph nodes isolated when an SP was applied. As neither the surgical technique nor the surgeons changed after the introduction of the SP, these results can be interpreted as a result of more meticulous analysis of the surgical specimens. Likewise, in our study we have observed a higher prevalence of pN + patients and those with microvascular invasion after the introduction of the SP.
Another important aspect to consider is the definition of R0/R1 margins. Most European groups define R1 resections as those where the tumor is less than 1 mm from the RM, while US pathologists consider R1 resection to be a tumor directly in contact with the RM. The impact of the distance between the tumor and the RM was analyzed by John et al.,23 who found differences in OS when the R1 resection was defined as tumors located less than 1 mm from the RM. Chang et al.,30 Jamieson et al.14 and Gebauer et al.25 analyzed the same relationship and found differences in OS when the tumor was located less than 1.5 or 2 mm. These data suggest that the definition of R1 should include tumors located at least 1 mm from the RM. Multicenter studies are needed to assess whether this limit should be increased to 1.5 or 2 mm.
The impact of RM involvement on DFS was investigated by Sugiura et al.31 and John et al.,23 who found no significant differences in DFS. Other series,3,19,21,24,32–35 however, including a more recent multicenter study,35 have demonstrated a significant decrease in both DFS and OS correlating with RM involvement. These findings are supported by our series, which demonstrates an increase in RR and a decrease in DFS and OS in patients with RM involvement after evaluation with the SP.
Regarding the influence of the involvement of each margin on the oncological prognosis, Delpero et al.18 and Ghaneh et al.35 demonstrated poorer OS rates with medial RM involvement. Our data corroborate the findings of a poorer oncological prognosis in cases with medial RM involvement, and they show a decrease in the DFS and OS in cases with involvement of the posterior RM, which in our series did not reach statistical significance. These findings have an important clinical relevance, since the increase in the R0 resection rate of the medial RM could be improved by neoadjuvant treatments or more aggressive surgical strategies with vascular resections in patients with unconfirmed SMV invasion. This situation is complex, since there is no clear evidence about whether resectable tumors with contact of less than 180° of the SMV in preoperative imaging tests should undergo neoadjuvant therapy as an initial treatment. In addition, the intraoperative evaluation of SMV invasion can be very complex due to the difficult differentiation between peritumoral fibrosis and tumor invasion itself. Our data suggest a poorer prognosis in cases of medial RM involvement; however, clinical trials are needed to find the best way to reduce this R1 rate.
There are limitations to our study, such as the extended time interval during which patients were recruited and changes to treatment strategies and chemotherapy regimens used throughout the study. Another limitation is the small number of patients included in our study, which has not allowed us to analyze the study variables in groups paired by possible confounding factors. Therefore, in our opinion, once the definition for different pancreatic margins and R1 resection are standardized, prospective multicenter studies are needed to evaluate the influence of neoadjuvant and adjuvant therapy as well as the aggressiveness of surgical strategies to improve the percentages of R0, DFS and OS.
In conclusion, after implementing an SP for the study of PDAC, our study shows an increase in the percentage of R1 resection, as well as a greater number of isolated lymph nodes, N + and microvascular invasion with the use of a standardized pathology report. The R1 resections in the SP group appear to show a higher RR and a reduction in both DFS and OS, which only maintained positivity for the medial RM when each of the RM were analyzed separately.
AuthorshipMDM: concept and design, data collection and analysis, composition, review; JLMN: data collection and manuscript review; MGR: data collection, image editing; EAM: manuscript review; EMP: concept, review, final approval.
Conflict of InterestsThe authors certify that there is no conflict of interests with research/academic funding regarding the content analyzed in the manuscript.
Please cite this article as: di Martino M, Muñoz de Nova JL, Guijarro Rojas M, Alday Muñoz E, Martín-Pérez E. Margen de resección positivo tras la aplicación de un examen estandarizado de la muestra de duodenopancreatectomía cefálica: ¿tiene un impacto real en la supervivencia a largo plazo? Cir Esp. 2020;98:127–135.