The objective of this study was to assess the diagnostic performance of combined computerised tomography (CT) and positron emission tomography (PET) in mediastinal staging of surgical lung cancer based on data obtained from the prospective cohort of the Spanish Group for Video-Assisted Thoracic Surgery (GEVATS).
MethodsA total of 2782 patients underwent surgery for primary lung carcinoma. We analysed diagnostic success in mediastinal lymph node staging (cN2) using CT and PET. Bivariate and multivariate analyses were performed of the factors involved in this success. The risk of unexpected pN2 disease was analysed for cases in which an invasive testing is recommended: cN1, the tumour centrally located or the tumour diameter >3 cm.
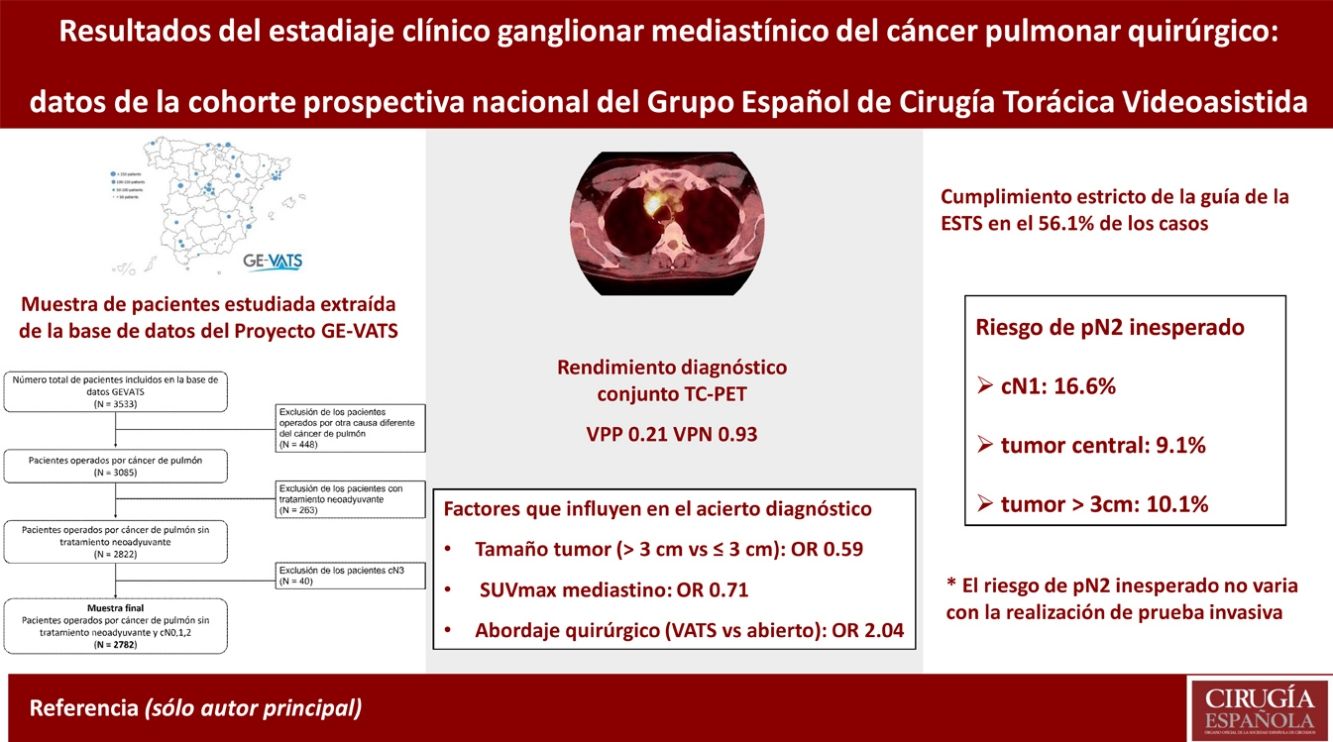
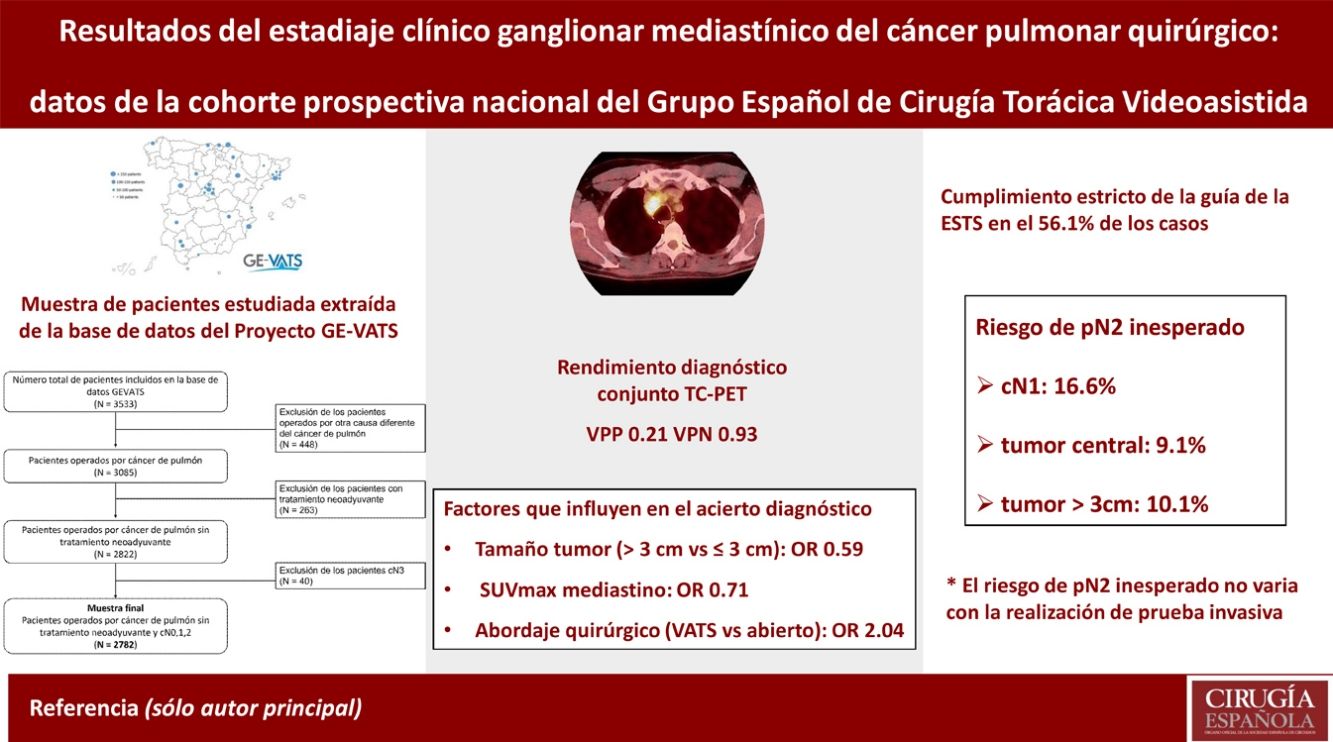
ResultsThe overall success of CT together with PET was 82.9% with a positive predictive value of 0.21 and negative predictive value of 0.93. If the tumour was larger than 3 cm and for each unit increase in mediastinal SUVmax, the probability of success was lower with OR 0.59 (0.44–0.79) and 0.71 (0.66–0.75), respectively. In the video-assisted thoracic surgery (VATS) approach, the probability of success was higher with OR 2.04 (1.52–2.73). The risk of unexpected pN2 increased with the risk factors cN1, the tumour centrally located or the tumour diameter >3 cm: from 4.5% (0 factors) to 18.8% (3 factors) but did not differ significantly as a function of whether invasive testing was performed.
ConclusionsCT and PET together have a high negative predictive value. The overall success of the staging is lower in the case of tumours >3 cm and high mediastinal SUVmax, and it is higher when VATS is performed. The risk of unexpected pN2 is higher if the disease is cN1, the tumour centrally located or the tumour diameter >3 cm but does not vary significantly as a function of whether patients have undergone invasive testing.
El objetivo del estudio es valorar el rendimiento diagnóstico de la tomografía computarizada (TC) y la tomografía por emisión de positrones (PET) en el estadiaje clínico mediastínico del cáncer pulmonar quirúrgico según los datos de la cohorte prospectiva del Grupo Español de Cirugía Torácica Videoasistida (GEVATS).
MétodosSe han analizado 2.782 pacientes intervenidos por carcinoma pulmonar primario. Se ha estudiado el acierto diagnóstico en el estadiaje mediastínico (cN2). Se ha realizado un análisis bivariante y multivariante de los factores que influyen en el acierto. Se ha estudiado el riesgo de pN2 inesperado en los factores con los que se recomienda una prueba invasiva de estadiaje: cN1, tumor central o tamaño mayor de 3 cm.
ResultadosEl acierto global de la TC y PET en conjunto es del 82,9% con VPP y VPN de 0,21 y 0,93. En tumores mayores de 3 cm y a mayor SUVmax del mediastino, el acierto es menor, OR de 0,59 (0,44–0,79) y 0,71 (0,66–0,75), respectivamente. En el abordaje VATS el acierto es mayor, OR de 2,04 (1,52–2,73). El riesgo de pN2 inesperado aumenta con el número de los factores cN1, tumor central o tamaño mayor de 3 cm: entre el 4,5% (0 factores) y 18,8% (3 factores), pero no hay diferencias significativas con la realización de prueba invasiva.
ConclusionesLa TC y PET en conjunto tienen un elevado valor predictivo negativo. Su acierto global es menor en tumores mayores de 3 cm y SUVmax del mediastino elevado, y mayor en el abordaje VATS. El riesgo de pN2 inesperado es mayor si cN1, tumor central o mayor de 3 cm y no varía significativamente con prueba invasiva.
Clinical mediastinal nodal staging (cN2) is a key element in the diagnostic process of lung cancer. When clinical nodal staging is cN0 or cN1, surgery is usually the initial treatment.1
Currently, the initial tests in mediastinal staging are computed tomography (CT) and positron emission tomography (PET). Depending on their results, it is decided whether invasive tests such as echo bronchoscopy (EBUS) or mediastinoscopy2 are necessary.
The combination of CT and PET has led to an improvement in staging as both have limitations individually.3 There are circumstances that may alter their negative predictive value (NPV) such as a central tumour, cN1 involvement or tumour size greater than 3 cm.4–8 One of the reference guidelines for the management of mediastinal staging published by the European Society for Thoracic Surgery (ESTS) in 20071 and subsequently updated in 2014,9 recommends invasive testing in these circumstances.
The aim of this study is to assess the diagnostic performance of CT and PET together in the clinical mediastinal staging of surgical lung cancer according to data obtained from the prospective cohort of the Spanish video-assisted thoracic surgery group (GEVATS).10
MethodsPatientsThe GEVATS project of the Spanish Society of Thoracic Surgery (SECT) was founded in May 2015 with the idea of studying the implementation of the VATS surgical approach in our country. A prospective multicentre cohort study was designed to include all anatomical lung resections (regardless of surgical approach) performed in the 33 centres that participated in 15 months (20/12/2016–20/03/2018). The research project was approved by all ethics committees of the participating centres and informed consent was obtained from the recruited patients for the use of clinical data for scientific purposes. All details on database characteristics, audit methods and variables are explained in the GEVATS10 study overview publication.
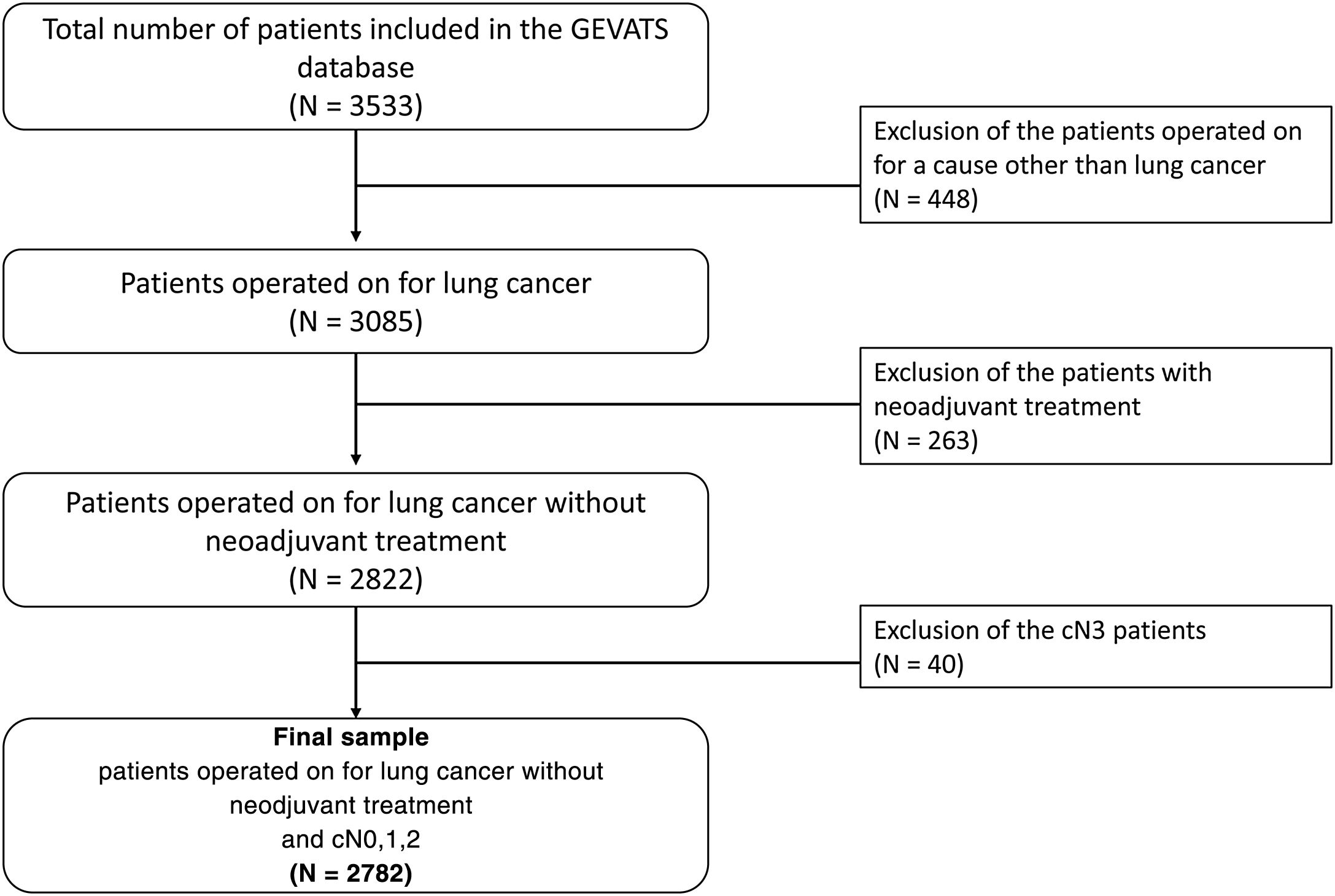
The database contains a total of 3533 patients of whom 3085 (87.3%) underwent surgery for lung carcinoma (Fig. 1). We excluded those who received neoadjuvant treatment, as it is impossible in these cases to distinguish whether the changes between clinical and pathological staging are due to errors in diagnostic tests or to the effects of treatment. We also excluded patients with a cN3 staging because they were lymph node areas not usually explored in surgery.
VariablesCT and PET were considered positive if any affected mediastinal lymph node (cN2) was detected in the test. In CT, a lymph node larger than 1 cm was considered positive and in PET, positivity was determined by each investigator based on the reference values of their centre. The variable clinical N2 (cN2) was defined by combining the CT and PET results. This variable is positive if either test is positive and negative if both tests are negative. In cases where PET was not performed, only the CT result was taken into account for the variable cN2. The hit variable was defined by comparing the clinical mediastinal stage cN2 and the pathological stage (pN2).
For the gold-standard assessment comparing the CT and PET diagnosis, the lymphadenectomy performed in the surgical procedure, the number of total lymph nodes removed and the number of stations explored are shown.
To assess the degree of compliance with the ESTS clinical guidelines, the variable compliance was developed. The criteria were those set out in the 20149 publication. If CT or PET scans are positive, an invasive test is required for histological confirmation. If they are negative and it is cN0, peripheral tumour and less than 3 cm in size, invasive testing should not be performed. In case of the presence of one of these three factors, despite being negative CT and PET, an invasive test must be performed.
ResultsThe diagnostic success and performance of CT and PET together and the factors influencing this success were analysed. The percentage of compliance with the ESTS guideline was calculated. A detailed study of the three factors where the ESTS guideline recommends invasive testing was performed. The risk of unexpected pN2 (cN2 negative and pN2 positive) in these cases was analysed and compared according to invasive testing.
Statistical analysisA descriptive analysis was initially performed. For quantitative variables, the mean and standard deviation (SD) or median and interquartile range were calculated. For qualitative variables, absolute and relative frequencies were calculated as a percentage.
We performed the Chi-square test or Fisher’s test to compare the distribution of qualitative variables. Similarly, we used Student’s t-test or Mann Whitney U-test, or ANOVA or Kruskall Wallis, to compare quantitative variables. A bivariate analysis was performed to determine the variables related to success. Those variables with a p-value < .20 were incorporated into a multivariate logistic regression model.
To assess the diagnostic performance of CT and PET versus gold-standard, Sensitivity (S), Specificity (S), Positive Predictive Value (PPV) and Negative Predictive Value (NPV) were calculated using point estimates and 95% confidence intervals.
Calculations were performed with STATA 16.18 (1985–2019 StataCorp LLC. Texas).
ResultsThe main demographic and clinical variables of the patient sample studied are contained in Table 1.
Demographic and clinical characteristics of the patient sample studied.
| Age (years) | 65.8 (9.6) |
| Gender | |
| Male | 1977 (71.1) |
| Female | 804 (28.9) |
| BMI | 26.9 (4.6) |
| Tobacco habit | |
| Never | 360 (12.9) |
| Ex-smoker 1–12 months | 1177 (42.3) |
| Ex-smoker > 12 months | 358 (12.9) |
| Smoker | 844 (30.4) |
| Unknown | 41 (1.5) |
| DM | 543 (19,5) |
| Surgical approach | |
| Open | 1216 (43.7) |
| VATS | 1566 (56.3) |
| 3 or more doors | 418 (26.7) |
| Bi-portal | 1005 (64.2) |
| Uni-portal | 140 (8.9) |
| Others | 3 (0.2) |
| Type of resection | |
| Segmentectomy | 163 (5.9) |
| Lobectomy | 2446 (87.9) |
| Pneumonectomy | 173 (6.2) |
| Type of lobectomy | |
| RUL | 855 (36.5) |
| ML | 138 (5.9) |
| RLL | 420 (17.9) |
| LUL | 561 (23.9) |
| LLL | 369 (15.7) |
| Tumour histology | |
| ADC | 1520 (54.7) |
| SCC | 855 (30.8) |
| TC | 152 (5.5) |
| AC | 38 (1.4) |
| LCNEC | 89 (3.2) |
| SCLC | 17 (0.6) |
| Undifferentiated | 43 (1.5) |
| Others | 63 (2.3) |
Data are shown as mean (SD) or absolute number (percentage).
AC: atypical carcinoid; ADC: adenocarcinoma; BMI: body mass index; LCNEC: large cell neuroendocrine carcinoma; LII: left lower lobe; LUL: left upper lobe; ML: middle lobe; RLL: right lower lobe; RUL: right upper lobe; SCC: squamous cell carcinoma; SCLC: small cell lung carcinoma; TC: typical carcinoid; VATS: video-assisted thoracic surgery.
A positive cN2 was detected in 381 (13.7%) patients and a pN2 in 253 (9.2%) (Table 2). The overall success of CT and PET together was 82.9%, with the most common error being cN2 positive (78.5%) (Table 2). S, E, PPV and NPV values were 0.32, 0.88, 0.21 and 0.93 respectively.
Description of clinical-pathological mediastinal staging.
| TC | |
| Negative (cN0/cN1) | 2578 (92.7) |
| Positive (cN2) | 203 (7.3) |
| PET | |
| Negative (cN0/cN1) | 2298 (88.6) |
| Positive (cN2) | 296 (11.4) |
| cN2 (TC + PET) | |
| Negative (cN0/cN1) | 2400 (86.3) |
| Positive (cN2) | 381 (13.7) |
| pN2 | |
| Negative (pN0/pN1) | 2489 (90.8) |
| Positive (pN2) | 253 (9.2) |
| Success in cN2a | 2273 (82.9) |
| Success | |
| pN2+/cN2+ | 81 (21.5) |
| pN2−/cN2− | 2192 (92.7) |
| No success | |
| pN2−/cN2+ | 296 (78.5) |
| pN2+/cN2− | 172 (7.3) |
Data are shown as absolute number (percentage).
cN (0,1,2): clinical nodal staging; CT: computed tomography; PET: positron emission tomography; pN (0,1,2): pathological nodal staging;
At lymphadenectomy, the mean number of total lymph nodes removed and total lymph node stations explored was 8.7 (SD 6.1) and 2.5 (SD 1.1).
The factors significantly influencing success in the bivariate analysis are shown in Tables 3 and 4. In the multivariate analysis, tumour size, mediastinal SUVmax and surgical approach maintained statistical significance (Table 5).
Univariate analysis of the factors (qualitative variables) influencing the diagnostic success of mediastinal nodal staging of CT and PET together.
| Variable | Diagnostic successa | P value |
|---|---|---|
| Tobacco habit | .070 | |
| Never | 308 (86.8) | |
| Ex-smoker | 1238 (81.7) | |
| Active smoker | 694 (83.3) | |
| DM | .042 | |
| NO DM | 1841 (83.6) | |
| YES DM | 431 (79.9) | |
| Tumour size | <.001 | |
| < = 3 cm | 1491 (87.8) | |
| >3 cm | 777 (75.1) | |
| CT tumour density | <.001 | |
| Solid | 1877 (81.5) | |
| Mixed | 286 (87.7) | |
| Ground-glass | 95 (97.9) | |
| Tumour location | <.001 | |
| Central | 793 (77.8) | |
| Peripheral | 1480 (85.9) | |
| Type of resection | <.001 | |
| Segmentectomy | 145 (91.8) | |
| Lobectomy | 2015 (83.4) | |
| Pneumonectomy | 113 (67.7) | |
| Type of lobectomy | .320 | |
| RUL | 701 (82.9) | |
| ML | 117 (87.9) | |
| RLL | 357 (85.8) | |
| LUL | 459 (82.7) | |
| LLL | 312 (85.5) | |
| Surgical approach | <.001 | |
| Thoracotomy | 901 (76.1) | |
| VATS | 1369 (88.1) |
P value < .05 in bold.
CT: computerised tomography; DM: diabetes mellitus; LII: left lower lobe; LUL: left upper lobe; ML: middle lobe; PET: positron emission tomography; RLL: right lower lobe; RUL: right upper lobe; VATS: video-assisted thoracic surgery.
Univariate analysis of the factors (quantitative variables) influencing the diagnostic success of mediastinal nodal staging of CT and PET together.
| Variable | Successful diagnosisa | Unsuccessful diagnosisa | P-valueb |
|---|---|---|---|
| Tumour size (mm) | 28.3 (19.7) | 38.7 (22.9) | <.001 |
| SUVmax tumor | 8.7 (6.8) | 12.3 (8.4) | <.001 |
| SUVmax mediastinal | 1.1 (2.2) | 3.4 (3.1) | <.001 |
| Nodal stations | 2.5 (1.1) | 2.6 (1.2) | .042 |
| Number of nodes | 8.4 (5.9) | 10.3 (7.1) | <.001 |
CT, computed tomography; PET, positron emission tomography; SUVmax, maximum standardized uptake value.
Multivariate analysis of factors influencing diagnostic success of mediastinal nodal staging of CT and PET together.
| Variablea | OR | 95% IC | P-value |
|---|---|---|---|
| Tumour size (>3 cm VS ≤ 3 cm) | .59 | .44–.79 | <.001 |
| SUVmax mediastinal | .71 | .66–.75 | <.001 |
| Surgical approach (VATS VS thoracotomy) | 2.04 | 1.52–2.73 | <.001 |
CI: confidence interval; CT, computed tomography; OR: odds ratio; PET: positron emission tomography; SUVmax: maximum standardized uptake value. VATS, video-assisted thoracic surgery.
ESTS nodal staging guidelines were adhered to in 1561 cases (56.1%). The least compliant cases were those with negative CT and PET scans and any of the three factors in which invasive testing is recommended.
When cN2 was positive, invasive testing was performed in 77.2% of patients compared to 13.5% in negative patients (Table 6). In cN2 negative cases, there was a relationship between the number of the three factors and the frequency of invasive testing: 0 (5.6%), 1 (12.4%), 2 (26.6%) and 3 (49.5%), a statistically significant difference (p < .001). The frequency of unexpected pN2 was higher if any of these 3 factors were present (Table 6). A relationship was also observed between the number of factors and the frequency of unexpected pN2: 0 (4.5%), 1, (7.7%), 2 (10.5%) and 3 (18.8%), a statistically significant difference (p < .001). No statistically significant differences were observed in the frequency of unexpected pN2 in these factors between patients with and without invasive testing (Table 6).
Invasive testing and pN2 as a function of cN2 and the three factors where invasive testing is recommended with negative cN2.
| Invasive testing (IT) | pN2 | pN2 without IT | pN2 with IT | P value | |
|---|---|---|---|---|---|
| cN2− | 323 (13.5) | 172 (7.3) | 143 (7) | 29 (8.9) | .208 |
| cN2+ | 294 (77.2) | 81 (21.5) | |||
| cN2− and cN0, peripheral tumour and ≤3 cm | 60 (5.6) | 47 (4.5) | |||
| cN2− and cN1 | 119 (39) | 50 (16.6) | 32 (17.5) | 18 (15.1) | .590 |
| cN2− and central tumour | 182 (21.5) | 76 (9.1) | 58 (8.9) | 18 (9.9) | .689 |
| cN2− and tumour >3 cm | 179 (21.7) | 82 (10.1) | 68 (10.7) | 14 (7.8) | .252 |
Data are shown as absolute number (percentage).
P-value for comparison of unexpected pN2 with IT and without IT.
cN1: clinical N1; cN2: clinical N2 (variable with CT and PET); IT: invasive test; pN2: pathological N2.
In the present study we analysed the diagnostic performance of CT and PET together in mediastinal lymph node staging of surgical lung cancer. The overall hit probability was 82.9%. The error is more frequent when these tests are positive for mediastinal involvement. When cN2 was positive, 21.5% of pN2 was detected and when negative, 7.3%. PPV and NPV values were .21 and .93 respectively. In view of the data, we reaffirm that a positive value on CT or PET for a mediastinal node requires further investigation by invasive testing. However, the low sensitivity observed is distorted by the fact that this is a surgical cohort.
The diagnostic performance of CT and PET alone is inferior as shown by Verhagen et al with a NPV of .83.4 Individually, PET appears to be more accurate.11 When both techniques are used together the performance improves.12 Wang et al published a meta-analysis with 10 studies and 1122 patients in which they estimated a NPV of .93 and 7% unexpected pN2 in stages cT1-2N0.7 One of these studies, performed only with surgical patients, observed an NPV and unexpected pN2 of .92 and 7.6% in cIA stages, which changes to .85 and 14.8% in cIB8 stages.
In these studies, the reference test for diagnostic success is often mentioned, but not its quality calidad.4,5,7,8,11,12. It would be interesting to have as detailed a description as possible of this test as proposed by Detterbeck et al.13 Some authors have observed an increase in the detection of pN2 when the extent of the lymph node dissection is greater.14 The Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) and other international guidelines recommend performing a systematic lymph node dissection with a minimum extraction of 6 nodes in total and at least 3 mediastinal stations explored.15–17 In our series, the mean number of nodes removed was 8.7 and 2.5 nodal stations explored.
Regarding the factors influencing the success of CT and PET, if the tumour is larger than 3 cm and the higher the SUVmax of the mediastinum, the probability of success is lower and in cases of VATS approach the probability of success is higher. These results are influenced by the lower degree of success in positive cN2. 21.7% of tumours larger than 3 cm have a positive cN2 compared to 8.6% in tumours smaller than or equal to 3 cm and the SUVmax of the mediastinum in cN2 positive cases is 3.5 points higher (data not presented in the results). In contrast, 19.3% of cases operated by thoracotomy have a positive cN2 vs. 9.4% of cases operated by thoracotomy have a positive cN2 vs. 9.4% in VATS cases. Also, the fact that VATS is less thorough in this approach, as demonstrated by Obiols et al in a recently published study using the same GEVATS18 series, may have an influence on the greater success of VATS.
The lower success of a positive CT or PET scan result means that invasive tests are more likely to be performed in such cases. In cases of negative results, the variability of action increases. In our series, the ESTS clinical practice guidelines were only strictly adhered to in 56.1% of cases. The situations of least compliance were with negative cN2 and cN1, central tumour or tumour larger than 3 cm. In these cases the ESTS guidelines recommend invasive testing and in our case this was only performed in 39%, 21.5% and 21.7% of cases respectively. The reason for indicating an invasive test is that the frequency of unexpected pN2 in these situations is higher. But even with surgical exploration of the mediastinum in 75% of patients, as in the work of Obiols et al, the unexpected pN2 was 5.5%19, slightly lower than the 7.3% observed in our study.
One of the most consistently published risk factors for unexpected pN2 is tumour size.7,20–24 Another factor that has been shown to be influential is the central location of the tumour. 20 However, the consistency of this factor is lowerr7,8 The cN1 has also shown a higher frequency of unexpected pN2.23,24 Hishida et al found 28% of pN2 patients with cN1 diagnosed by CT.24
Apart from the individual risk of these factors, it is important to assess the risk of unexpected pN2 when several of them are combined. In Farjah’s work, they designed a predictive model with 6 factors with a NPV of 100%.23. In our series, we have observed that the percentage of unexpected pN2 is higher the greater the number of the three risk factors studied, rising from 4.5% in the case of none to 18.8% when all three are present. This should therefore be taken into account when deciding whether to perform invasive testing.
The performance of these tests in the staging of surgical lung cancer is the point of greatest variability. Thornblade et al observed large differences in invasive staging between centres, not explained by tumour stage.25 It is important to identify cases where invasive testing is not necessary and we can save morbidity and study time. In a model combining several factors, they estimate that the number of invasive tests could be reduced from 77% to 55%.23 In another cost-effectiveness study26 they conclude that below 2.5% pN2 it is cost-effective not to perform invasive testing and between 2.5 and 10% each case must be assessed individually. In our series, with a prevalence of pN2 of 18.8% with the presence of the 3 risk factors, invasive testing was only performed in 49.5% of cases. However, invasive testing did not significantly reduce the risk of unexpected pN2.
The criteria used in the literature to recommend invasive mediastinal staging do not take into quality-adjusted years on survival data published in the literature and not on the direct effects of a staging strategy.26–28 Furthermore, Decaluwé et al. reflect that we should know the survival benefit of administering neoadjuvant treatment in an N2 prior to surgery versus administering adjuvant treatment after finding an unexpected pN2.29 Based on literature data, he estimates that between 580 and 2900 invasive tests would be necessary to save a life at 5 years. In our cohort, which is currently under follow-up, we will be able to see the impact on survival at 5 years of the unexpected finding of a pN2 when no invasive test has been performed compared to those patients who have received neoadjuvant treatment for a cN2 at the same time.
Several limitations can be observed in our study. Firstly, as this is a voluntary registry, we may have doubts about the quality of the data. However, the audit and quality control carried out in this registry have allowed us to verify an average recruitment rate of 83% and data success of 98%, which makes the information obtained reliable.10 On the other hand, like all studies that analyse this subject, we have the limitation of having a variable gold-standard and without exact and agreed quality criteria. We have shown the number of nodes and stations as an assessment of the gold standard. In any case, our data show the performance of non-invasive clinical staging in routine clinical practice with the types and extensions of lymphadenectomy usually performed in our setting.
ConclusionsCT and PET together have a high diagnostic yield in mediastinal lymph node staging in surgical lung cancer in cases with a negative result for cN2. Their overall success is lower in the case of tumours larger than 3 cm and high mediastinal SUVmax, and is higher in the case of VATS surgical approach.
When CT and PET are negative for cN2 but cN1, central tumour or larger than 3 cm, the risk of unexpected pN2 is higher and increases with the number of these factors, but there is no significant variation with invasive testing.
FinancingAll costs related to the set-up and maintenance of the GEVATS database were covered by Ethicon, Johnson & Johnson. The authors had research freedom and full control over the study design, methods used, outcome parameters and results, data analysis and the production of the written report. GEVATS was awarded the SECT award for the best national research project in 2015.
Conflict of interestsThe authors have no conflict of interests to declare directly or indirectly related to the manuscript contents.
Our thanks to Johnson & Johnson for their collaboration in the development of the Spanish VATS group. Our thanks too to all those responsible for the clinical documentation of each hospital for actively participating in the auditing of our study.
Please cite this article as: Lopez I, Aguinagalde B, Urreta I, Royo I, Bolufer S, Sanchez L, et al. Resultados del estadiaje clínico ganglionar mediastínico del cáncer pulmonar quirúrgico: datos de la cohorte prospectiva nacional del Grupo Español de Cirugía Torácica Videoasistida. Cir Esp. 2022. https://doi.org/10.1016/j.ciresp.2022.05.001