Brunner gland hamartoma is an uncommon lesion that is usually located in the first portion of the duodenum. Its origin is submucosal and it has a potential risk for bleeding.
There are no specific symptoms nor are there any endoscopic images to aid in the initial diagnosis. Endoscopic ultrasound has been suggested as the gold standard method due to its capability to identify this pathology. We present a clinical case with CT diagnosis and its unique radiological characteristics, and we review the literature published to date.
The patient is a 40-year-old male with no history of interest who came to our hospital due to syncope and melenas.
He had had no previous episodes of gastrointestinal bleeding until 48h before, when the melenas had begun. The patient was haemodynamically stable, and the abdomen was soft and non-painful. Lab workup showed haemoglobin 10.3g/dL, HCT 29.6%, 13400 leukocytes and 81% PMN; the remaining haemogram and biochemistry levels were normal.
Gastroscopy revealed a polypoid lesion in the superior duodenal knee; gastrointestinal stromal tumour was ruled out, and no signs of previous bleeding were observed.
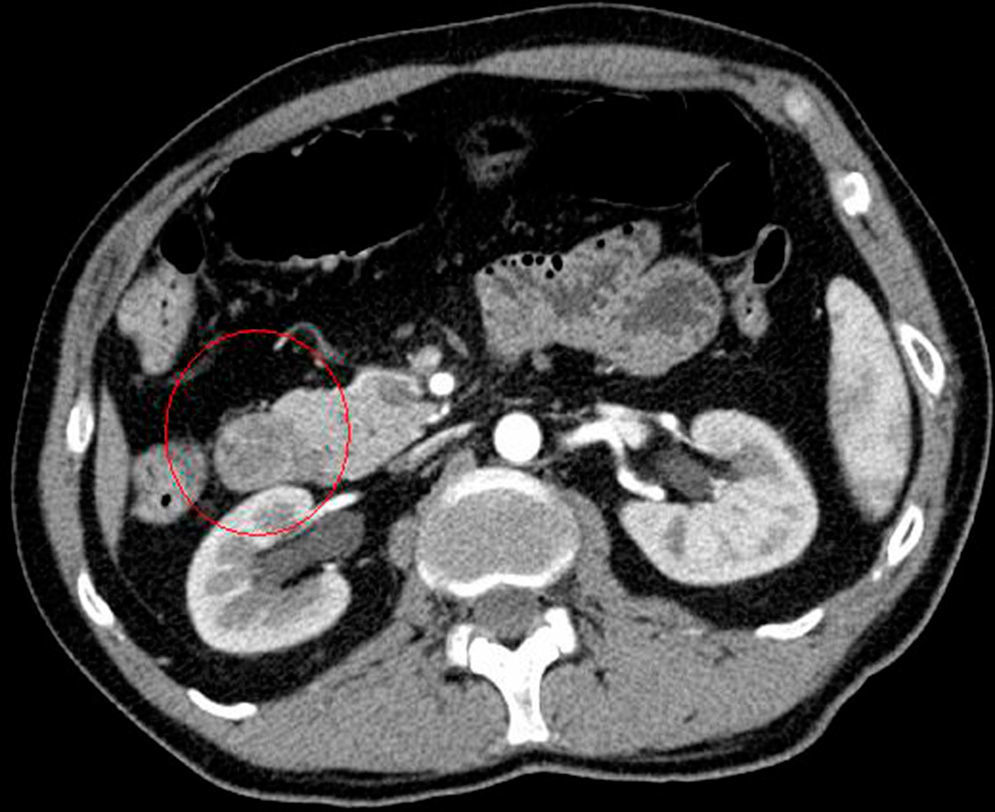
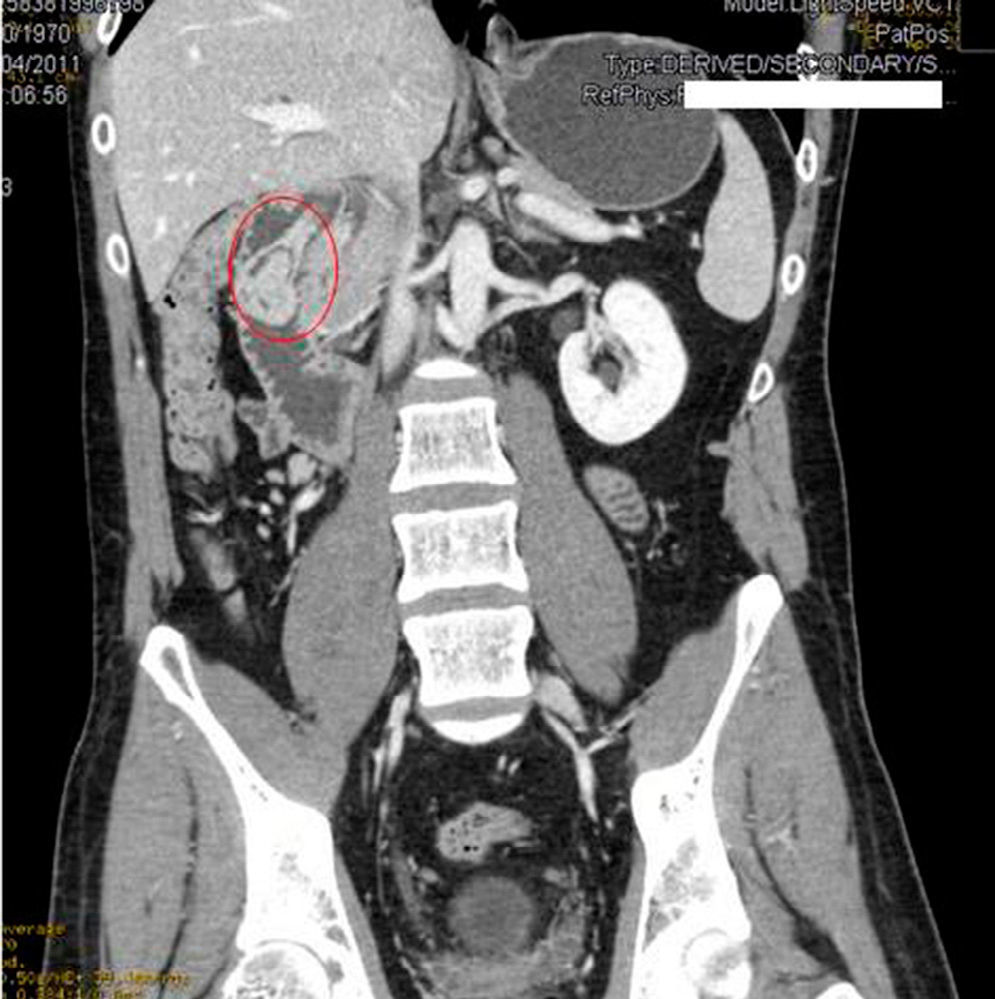
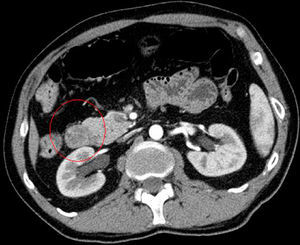
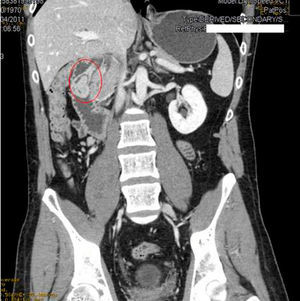
We requested an abdominal CT. The results reported the mass was a polypoid lesion measuring 3cm×2.6cm in the second portion of the duodenum, which had a 3cm pedicle that was implanted in the posterior side of the duodenal bulb. The lesion presented heterogenous uptake, with a peculiar presence of areas of fatty density in its interior, which provided a probable diagnosis of Brunner gland hamartoma (Figs. 1 and 2).
We performed gastroscopy with polypectomy and haemostatic control of the pedicle with adrenalin and endoclips.
The pathology study defined the lesion as a mucosal–submucosal mass of hyperplastic Brunner glands and observed proliferated hyperplastic glandular lobules with cystic dilatations, separated by fibrous tissue, adipocytes and smooth muscle fibres. These findings were compatible with Brunner gland hamartoma.
The patient was discharged. He continues to be asymptomatic, and endoscopic follow-up studies have shown good progress.
The Brunner glands are acinar structures that are mainly situated in the first portion of the duodenum, which diminish in number distally.1 Their function seems to be related with gastric acid, as their secretions inactivate the acidity of gastric juices.2
Since their discovery in 1688, the nomenclature of the lesions associated with their growth has gone through different stages. Currently, the lesions are considered hamartomas given the abnormal proliferation of morphologically normal tissues (without atypia) within the lesion.
It is a rare tumour with a reported approximate incidence of 1% of small bowel tumours and 5% of duodenal tumours.3 They are most frequently diagnosed between the 5th and 6th decades of life, with a slightly greater prevalence in men.1,2
The aetiology is not clear. Initially, it was proposed that their growth could possibly be a consequence of hyperchlorhydria, based on their function as an acid buffer, but this possibility was ruled out since the lesions are not more frequent in cases of Zollinger–Ellison syndrome. Furthermore, they do not recede with antisecretory treatment.
There seems to be a certain correlation with recurring pancreatitis episodes that lead to chronic pancreatitis, possibly as a mechanism for adapting to the exocrine insufficiency caused by these conditions.2
Clinically, most published case reports to date are based on the diagnosis as a consequence of upper gastrointestinal bleeding4 or intestinal obstruction. Indeed, it seems that what is most common is that the lesion, especially when it reaches a certain size (larger than 1cm), debuts with symptoms of upper gastrointestinal bleeding.2,5–7
On other occasions, the diagnosis can be incidental or due to symptoms of duodenal/intestinal obstruction, intussusception, non-painful jaundice or pancreatitis.
The diagnosis is histological. Endoscopic biopsy generally does not reach planes beyond the mucosa, which provides for visualisation of the hamartomatous tissue. Endoscopic ultrasound is useful for the diagnosis. And in most cases, a well-defined heterogenous hypoechoic submucosal nodule is observed.
In our case, it was not necessary to recur to endoscopic ultrasound because the CT scan was diagnostic. Hur et al.7 described the radiological–pathological association of Brunner gland hamartomas studied by CT. In general, the lesions are observed to be nodules in the first duodenal portion that are hypoattenuated compared to the pancreatic parenchyma and heterogeneous. The heterogeneity corresponds to the areas of adipose tissue and smooth muscle proliferation. On other occasions, where there is less amount of mesenchymal tissue, the lesion can be described as homogeneously hypoattenuated, but this is less frequent.
The differential diagnosis should include adenomatous polyps, gastrointestinal stromal tumours, lymphomas, carcinoid tumours and tumours of the pancreatic and periampullary areas.1,2,6
The clinical approach should be endoscopic and is recommended when symptoms arise. Surgery by means of duodenotomy or segmental resection should be reserved for cases that cannot be treated with endoscopy.2
The prognosis is excellent. Although cases of malignant degeneration of hamartomas have been described,3 these may actually be focal adenocarcinomas over a non-dysplastic Brunner hamartoma.
Please cite this article as: García-Marín JA, Lirón-Ruiz RJ, Girela-Baena EL, Aguayo-Albasini JL. Papel de la tomografía computarizada en el diagnóstico del hamartoma de las glándulas de Brunner. Cir Esp. 2016;94:e17–e19.