Solid pseudopapillary tumours of the pancreas are extremely rare epithelial tumours with a limited potential for malignancy. They represent less than 1%–2% of all exocrine pancreatic tumours.1 These neoplasms were initially described in 19592 and have been given different names since then: papillary tumour of the pancreas, Frantz tumour, solid cystic papillary epithelial neoplasm, or papillary cystic neoplasm, and since 1996 they have been called solid pseudopapillary tumour of the pancreas.3 They most frequently affect young woman of Asian or African descent between the ages of 20 and 40, although there have been isolated cases in children and in men.
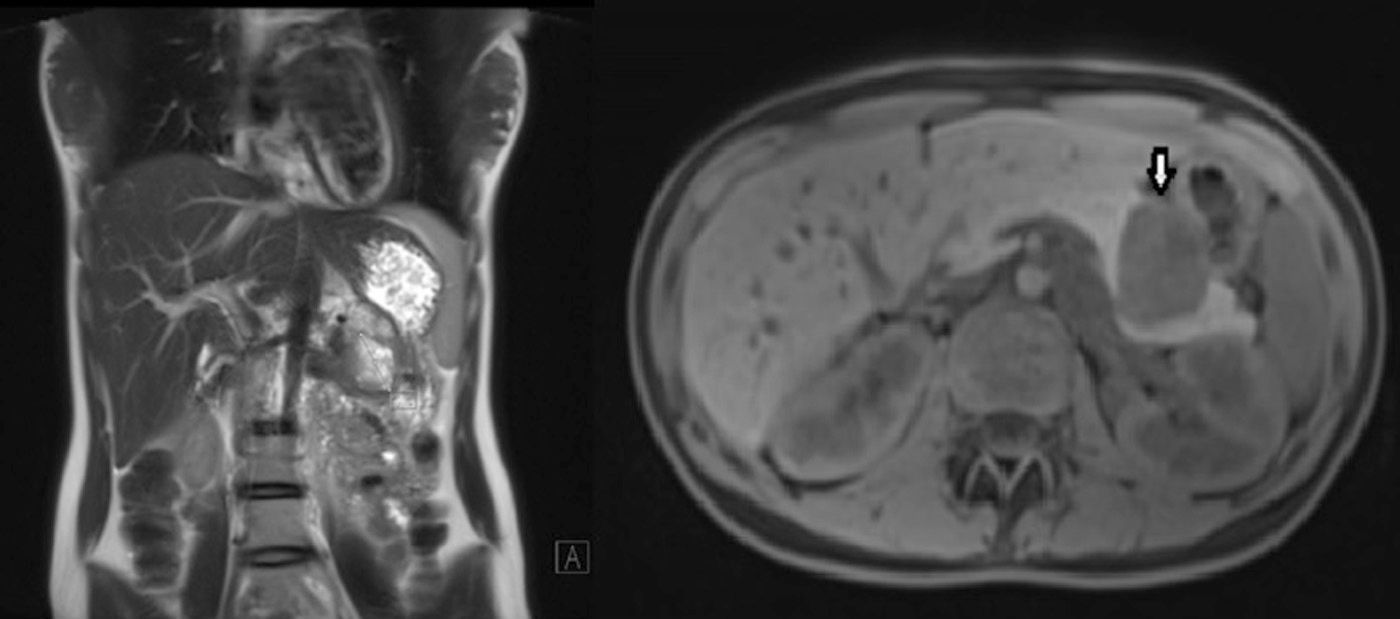
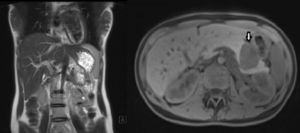
We present the case of a 17-year-old female patient who complained of epigastric abdominal pain and a feeling of early satiety that had been progressing over several months, with no other symptoms. Gastroscopy showed evidence of extrinsic gastric compression on the body of the stomach. Abdominal CT scan and MRI (Fig. 1) detected a 5cm solid retroperitoneal mass dependent on the body of the pancreas. Endoscopic ultrasound showed that it was a solid hypervascular lesion in the body/tail of the pancreas. FNA indicated the diagnosis of solid pseudopapillary neoplasm of the pancreas. The lab results, which included tumour marker levels, were within normal ranges.
Given the suspected diagnosis, laparoscopic distal pancreatectomy was performed with preservation of the spleen and splenic vessels (laparoscopic Mallet-Guy technique),4 without incident. The postoperative recovery was uneventful and the patient was discharged on the sixth day post-op.
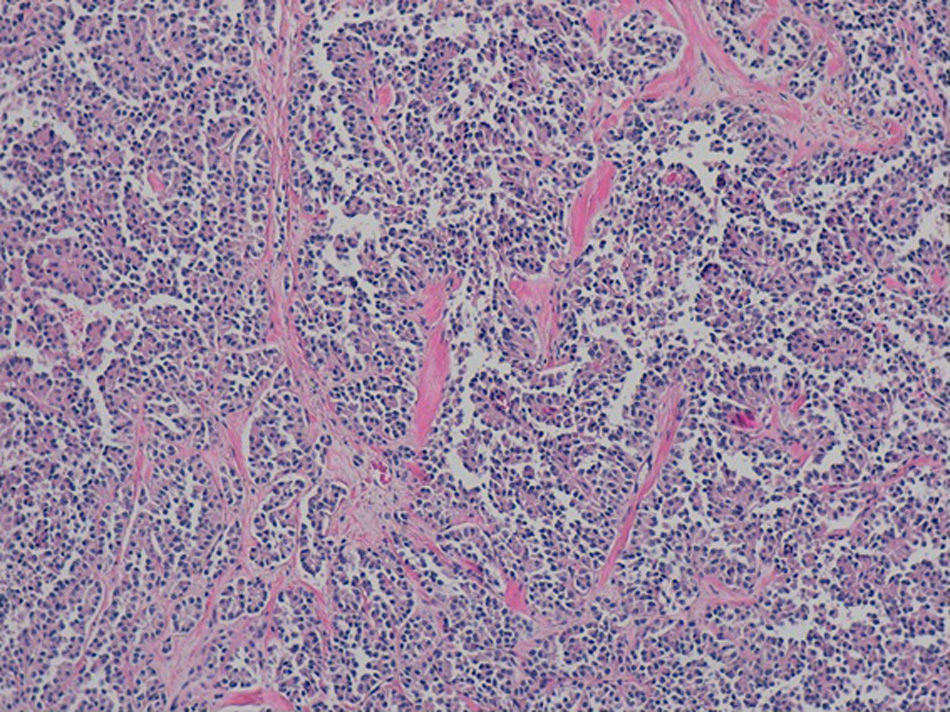
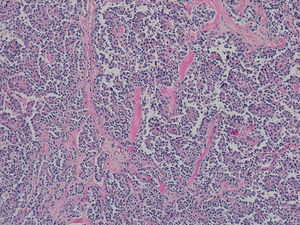
The definitive pathology analysis confirmed the diagnosis of pseudopapillary neoplasm of the pancreas, with no vascular or perineural invasion (Fig. 2). The immunohistochemistry study was strongly positive for CD56, CD10 and beta-catenin. Progesterone and synaptophysin showed focal positivity, and cytokeratin AE1–AE3 and chromogranin were negative.
Pseudopapillary tumours of the pancreas are very uncommon pancreatic neoplasms of unknown aetiology that mainly affect young women in the second and third decades of life. It has been proposed that their origin could be epithelial ductal, neuroendocrine, pluripotent stem cell and even extrapancreatic genital.5 Prognosis is favourable even in the presence of distant metastasis, and survival rates of more than 10 years have been described even in the presence of hepatic or peritoneal metastases.6 The clinical manifestations are nonspecific and related with tumour size, although they usually include abdominal pain, sensation of fullness or the presence of an abdominal mass.7
Laboratory analyses are usually normal and the most frequent location is the tail of the pancreas, followed by the body.8 Diagnosis is usually based on imaging tests (ultrasound, CT and MRI), which demonstrate a well-outlined mass that is encapsulated and heterogeneous (solid-cystic) with occasional calcifications and necrotic areas.9 The differential diagnosis must be done with cystadenoma, cystadenocarcinoma, mucinous cystic neoplasms, pancreatoblastomas, teratomas and pancreatic neuroendocrine tumours as the most frequent hypervascular lesions. The diagnosis should be suspected in hypervascular solid-cystic pancreatic lesions in young women and, in cases of doubt, FNA with endoscopic ultrasound can confirm the preoperative diagnosis.10,11 For the differential diagnosis with neuroendocrine tumours, most of which present somatostatin receptors, OctreoScan® could be used since solid pseudopapillary neoplasms lack this type of receptors.
The definitive diagnosis is determined by biopsy, and the recommended treatment is surgical resection. 85% of cases are limited to the pancreas at the time of diagnosis, and the remainder have metastasised at the time of diagnosis.
The most frequent locations of the metastases are the liver, regional lymph nodes, mesentery, omentum and peritoneum.
The treatment of choice is surgery; lymphadenectomy, however, is not recommended when the presentation is focalised. When there is metastasis or local invasion, surgery is still the treatment of choice. In the pathology analysis, there is a characteristic presence of solid areas alternating with pseudopapillary areas, although there is a recent report of increased nuclear and cytoplasmic expression of E-cadherin and beta-catenin as specific markers.12
The incidence of malignant solid pseudopapillary neoplasms or solid pseudopapillary carcinoma is 15%. Certain histological characteristics have been associated with an aggressive behaviour, such as high mitotic rate, nuclear atypia, extensive necrosis, sarcomatoid areas and expression of Ki-67.13 Moreover, Ki-67 has been proposed as an indicator of malignant potential, so that a low rate (below 5%) indicates slow tumour growth and better prognosis.14,15 The role of adjuvant radiotherapy or chemotherapy is not clear, although they are generally reserved for unresectable cases.
Nonetheless, although surgical resection is generally curative, follow-up is recommended to diagnose local recurrences and distant metastases. Overall 5-year survival is 95%.7,16,17
Please cite this article as: Jiménez-Fuertes M, Ramírez-García JR, Ruiz-Tovar J, Díaz García G, Durán-Poveda M. Neoplasia sólida pseudopapilar de páncreas. Cir Esp. 2016;94:e31–e33.