Renal cell carcinoma is responsible for 2%–3% of all malignant tumors in adults, with a higher incidence in men. The 5-year survival rate is 95% in tumors limited to the kidney, while the survival rate in disseminated tumors is 20%1. Some 20%–30% of patients have metastasis at diagnosis, and 50% will develop metastasis after nephrectomy. The most common locations are the lung, liver, bone, and brain, but it is also relatively common to find lesions in unusual places, such as the thyroid, skeletal muscle, and pancreas2.
The reported incidence of pancreatic metastases from renal cell carcinoma is 2%. They typically appear long after nephrectomy, with intervals even longer than 30 years. Preoperatively, they can be difficult to differentiate from other types of pancreatic lesions. The typical CT image is a hypervascular nodule with defined margins, whose main differential diagnosis includes pancreatic neuroendocrine tumors. Endoscopic ultrasound and biopsy of the lesion may be necessary. In some cases, its nature is only determined after immunohistochemical analysis of the surgical specimen. Therefore, with a history of renal cell carcinoma, it is important to have a high rate of suspicion when evaluating this type of lesion2,3.
Metastases in the pancreas are associated with a better prognosis than other locations. Patients with resectable metastases should be candidates for surgery, as this increases survival4.
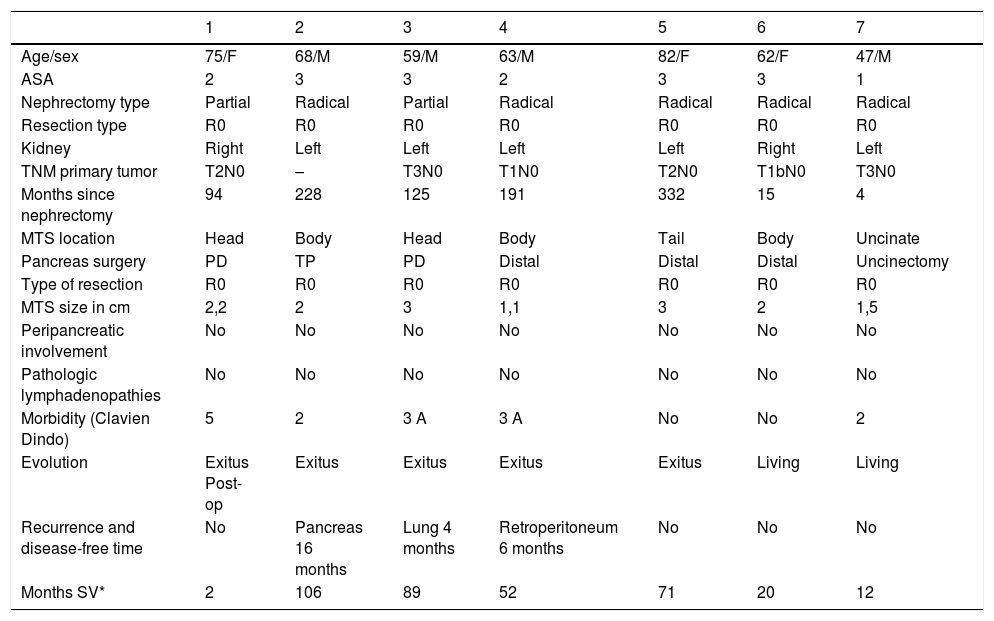
We conducted a retrospective analysis of 7 patients who were treated surgically at our hospital between 2000 and 2019 for pancreatic metastases of renal carcinoma. The variables studied are summarized in Table 1.
Clinical and demographic characteristics.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Age/sex | 75/F | 68/M | 59/M | 63/M | 82/F | 62/F | 47/M |
| ASA | 2 | 3 | 3 | 2 | 3 | 3 | 1 |
| Nephrectomy type | Partial | Radical | Partial | Radical | Radical | Radical | Radical |
| Resection type | R0 | R0 | R0 | R0 | R0 | R0 | R0 |
| Kidney | Right | Left | Left | Left | Left | Right | Left |
| TNM primary tumor | T2N0 | – | T3N0 | T1N0 | T2N0 | T1bN0 | T3N0 |
| Months since nephrectomy | 94 | 228 | 125 | 191 | 332 | 15 | 4 |
| MTS location | Head | Body | Head | Body | Tail | Body | Uncinate |
| Pancreas surgery | PD | TP | PD | Distal | Distal | Distal | Uncinectomy |
| Type of resection | R0 | R0 | R0 | R0 | R0 | R0 | R0 |
| MTS size in cm | 2,2 | 2 | 3 | 1,1 | 3 | 2 | 1,5 |
| Peripancreatic involvement | No | No | No | No | No | No | No |
| Pathologic lymphadenopathies | No | No | No | No | No | No | No |
| Morbidity (Clavien Dindo) | 5 | 2 | 3 A | 3 A | No | No | 2 |
| Evolution | Exitus Post-op | Exitus | Exitus | Exitus | Exitus | Living | Living |
| Recurrence and disease-free time | No | Pancreas 16 months | Lung 4 months | Retroperitoneum 6 months | No | No | No |
| Months SV* | 2 | 106 | 89 | 52 | 71 | 20 | 12 |
The categorical variables are expressed as percentage and quantitative variables with non-normal distribution as median and interquartile range.
PD: pancreaticoduodenectomy; F: female; M: male; MTS: metastasis; Post-op: postoperative; TP: total pancreatectomy.
Mean age was 63 years (IQR 59–75). The patients were 4 men and 3 women. The primary tumor was located in the left kidney in 5 patients and in the right kidney in 2 (Table 1).
None of the patients presented symptoms at diagnosis. The median latency between surgery of the primary tumor and the development of pancreatic metastases was 125 months (IQR 15–228) with a minimum of 4 months and a maximum of 332 months. Two patients (28.6%) had previously presented metastases in other organs (lung and oral cavity), and both cases were treated with R0 resection.
Regarding the surgical technique, we performed 4 distal pancreatectomies, 2 pancreaticoduodenectomies (PD) and one resection of the uncinate process, achieving R0 resection in all cases. Morbidity was 70% (40% Clavien Dindo II and another 40% grade IIIa). Regarding mortality, one of the 7 patients died 83 days after surgery due to heart failure.
Median survival was 5.9 years (IQR 2.25–8.1). Two of the 7 patients are still alive after follow-up periods of 20 and 12 months. The latency time to metastasis and the percentage of involvement of other organs were similar reports in the literature5.
No relationship has been demonstrated between the location of the primary tumor and the location of the metastasis; therefore, local venous or lymphatic spread seems unlikely. The dissemination mechanism appears to be hematogenous, with a certain affinity for the pancreatic parenchyma6.
Tanis et al. estimated that after resection of the pancreatic metastasis, the local recurrence rate was 4%, and the extrapancreatic recurrence rate was 17.1%3. In our experience, we found one case of recurrence in the pancreas that required completion of the pancreatectomy and two cases of distant recurrence (lung and retroperitoneal).
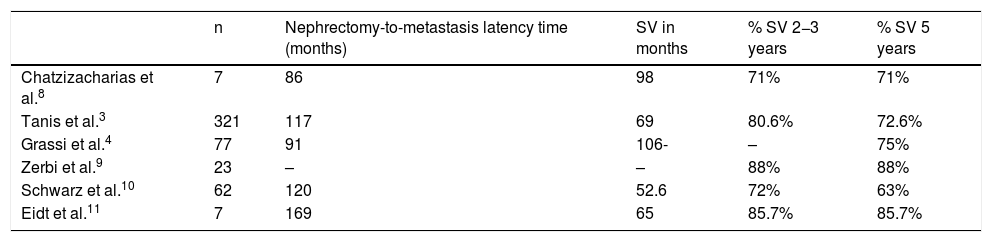
Most publications are short series and case reports, but a systematic review published in 2009 compared the survival of 321 patients who underwent pancreatic resection and another 73 in whom resection was not performed. The study showed an increase in 2-year and 5-year survival rates (80.6 and 72.6%) in the surgery group compared to the group without resection (41% and 14%)3,7 (Table 2).
Latency and survival times in the main series published.
| n | Nephrectomy-to-metastasis latency time (months) | SV in months | % SV 2−3 years | % SV 5 years | |
|---|---|---|---|---|---|
| Chatzizacharias et al.8 | 7 | 86 | 98 | 71% | 71% |
| Tanis et al.3 | 321 | 117 | 69 | 80.6% | 72.6% |
| Grassi et al.4 | 77 | 91 | 106- | – | 75% |
| Zerbi et al.9 | 23 | – | – | 88% | 88% |
| Schwarz et al.10 | 62 | 120 | 52.6 | 72% | 63% |
| Eidt et al.11 | 7 | 169 | 65 | 85.7% | 85.7% |
SV: survival.
These metastases usually appear as metachronous isolated metastases, although cases of two or more pancreatic metastases have been reported. The type of surgical treatment remains controversial. Some authors advocate atypical resections with the selective excision of the lesion to preserve the pancreatic endocrine and exocrine function, whereas other surgeons prefer standard pancreatectomies (PD, distal pancreatectomies and TP) in order to reduce pancreatic recurrences8,9.
In recent years, studies have been published with tyrosine kinase inhibitors, showing no statistically significant differences in survival versus surgery, but with lower survival curves10. Chemotherapy may be an option in patients who are not candidates for surgery due to either unresectable disease or comorbidities4.
In conclusion, pancreatic metastases of renal carcinomas are rare and can occur many years after the primary tumor, so this should be considered as a possible differential diagnosis. Pancreatic surgery should be considered in these patients as it increases survival.
Please cite this article as: Vilar Tabanera A, Muñoz Muñoz P, Molina Villar JM, Gajate P, Sanjuanbenito A. Cirugía en las metástasis pancreáticas por carcinoma renal. Cir Esp. 2022;100:50–52.