The first line of therapy in children with hypercholesterolaemia is therapeutic lifestyle changes (TLSC). The efficacy of lifestyle intervention in children with familial hypercholesterolaemia (FH), where LDL-C levels are genetically driven, deserves a focused study.
AimsTo evaluate the impact of a lifestyle education program, focused on food patterns and physical activity, on lipid profiles assessed by nuclear magnetic resonance (NMR) in children with FH vs. non-FH.
MethodsPhase 1 was a cross-sectional study of baseline characteristics, and phase 2 was a prospective TLSC intervention study. In total, the study included 238 children (4 to 18 years old; 47% girls) attending the lipid unit of our hospital due to high cholesterol levels. Eighty-five were diagnosed with FH (72% genetic positive), and 153 were diagnosed with non-Familial hypercholesterolaemia. A quantitative food frequency questionnaire (FFQ) including 137 items was used. Physical activity (PA) was assessed by the Minnesota questionnaire. The lipid profile was assessed using the 2D-1H-NMR (Liposcale test). A total of 127 children (81 in the FH group) participated in the prospective phase and were re-assessed after 1 year of the TLSC intervention, consisting of education on lifestyle changes delivered by a specialized nutritionist.
ResultsThe FH and non-FH groups were similar in anthropometry and clinical data, except that those in the FH were slightly younger than those in the non-FH group. Both the FH and non-FH groups showed a similar diet composition characterized by a high absolute calorie intake and a high percentage of fat, mainly saturated fat. The PA was below the recommended level in both groups. After one year of TLSC, the percentage of total and saturated fats was reduced, and the amount of fiber increased significantly in both groups. The percentage of protein increased slightly. The number of children engaged in at least 1 hour/day of PA increased by 56% in the FH group and by 53% in the non-FH group, and both these increases were significant. The total and small-LDL particle numbers were reduced in both groups, although the absolute change was greater in the FH group than in the non-FH group.
ConclusionsEducational strategies to implement TLSC in children lead to empowerment, increased adherence, and overall metabolic improvement in children with high blood cholesterol, including those with FH.
La primera línea de terapia en niños con hipercolesterolemia son los cambios terapéuticos en el estilo de vida (TLSC). La eficacia de la intervención en el estilo de vida en niños con hipercolesterolemia familiar (HF), en los que los niveles de LDL-C son generados genéticamente, merece un estudio específico.
ObjetivosEvaluar el impacto de un programa de educación sobre el estilo de vida, centrado en los patrones alimentarios y la actividad física, sobre el perfil lipídico evaluado por resonancia magnética nuclear (RMN) en niños con HF versus no HF.
MétodosLa fase 1 fue un estudio transversal de las características basales, y la fase 2 fue un estudio prospectivo de intervención mediante TLSC. En total, el estudio incluyó a 238 niños (de 4 a 18 años; 47% niñas) que asistieron a la unidad de lípidos de nuestro hospital debido a los altos niveles de colesterol. Ochenta y cinco fueron diagnosticados con HF (72% genéticamente positivos), y 153 fueron diagnosticados de no HF. Se utilizó un cuestionario cuantitativo de frecuencia de alimentos (FFQ) que incluye 137 ítems. La actividad física (AF) se evaluó mediante el cuestionario de Minnesota. El perfil lipídico se evaluó mediante la prueba 2D-1H-NMR (Liposcale Test). Un total de 127 niños (81 en el grupo HF) participaron en la fase prospectiva y fueron reevaluados después de 1 año de la intervención mediante TLSC, que consistió en educación sobre cambios en el estilo de vida impartida por una nutricionista especializada.
ResultadosLos grupos HF y no HF fueron similares en los datos antropométricos y clínicos, excepto que los HF eran ligeramente más jóvenes que los no HF. Los participantes de ambos grupos mostraron una composición de dieta similar caracterizada por un alto consumo de calorías totales y un alto porcentaje de grasas, principalmente grasas saturadas. La AF estuvo por debajo del nivel recomendado en ambos grupos. Después de un año de TLSC, se redujo el porcentaje de grasas totales y saturadas, y la cantidad de fibra aumentó significativamente en ambos grupos. El porcentaje de proteína aumentó ligeramente. El número de niños involucrados en al menos 1 hora/día de AF aumentó en un 56% en el grupo de HF y en un 53% en el grupo sin HF, y ambos aumentos fueron significativos. Los números de partículas LDL totales y pequeñas se redujeron en ambos grupos, aunque el cambio absoluto fue mayor en el grupo HF que en el grupo no HF.
ConclusionesLas estrategias educativas para implementar TLSC en niños conducen al empoderamiento, al aumento de la adherencia y a la mejora metabólica general en niños con colesterol alto en sangre, incluidos aquellos con HF.
Familial hypercholesterolemia (FH) is an autosomal-dominant disorder of lipid metabolism characterized by an increase in low-density lipoprotein cholesterol (LDL-C).1 The elevation of LDL is observed at an early age and results in early atherosclerotic lesions.2 This disease has been described as a cause of premature atherosclerotic coronary heart disease, and for this reason, it is very important to start treating children.3
In Catalonia, one out of 217 children has the FH phenotype.4 This disease is very prevalent worldwide and is underdiagnosed.2 In recent years, several studies have been published to improve the detection of FH.5–7 The need for improved detection arises from the need to start treatment earlier to improve the prognosis.1,8–10 In addition, early detection strategies are efficient.11–13
The first line of therapy in hypercholesterolemic children is therapeutic lifestyle changes (TLSC), and only FH children with very high LDL-C will require medication1,8,14,15 to achieve the LDL-C goal of 130mg/dL.2,8,16 Total cholesterol and LDL-C levels can be modulated by dietary intake.17–18 Studies have shown that dietary adjustments can reduce plasma cholesterol by 20–30%17 and LDL atherogenicity.19 The efficacy of lifestyle interventions in children with FH, where LDL-C levels are genetically driven, deserves focused study. Some authors have observed that the reduction in LDL-C in patients with FH is lower than in patients without FH.19–21 The effect of diet on the lipid profile of these children is little known.
The atherogenicity of LDL particles is not measured only by the concentration of cholesterol. The LDL particle number is considered an even better risk marker. Interestingly, small LDL particles are more atherogenic because they are more easily oxidizable, they can pass through the membrane, and they have less affinity for the receptor.22–23 Small LDL is viewed as an important cardiovascular risk factor.24–25 FH children exhibit a significant absolute increase in the LDL particle number, which includes the most atherogenic small LDL particles.26–27 These characteristics can be evaluated using metabolomics techniques based on nuclear magnetic resonance (NMR). Studies showing the effect of diet on lipoprotein particle number and size in FH children are scarce.
The purpose of the present study was to characterize the total lipid profile of FH and non-FH hypercholesterolemic children, to appraise the diet and PA of FH and non-FH hypercholesterolemic children and to prospectively evaluate the impact of a lifestyle intervention on food patterns, PA and lipid profiles of FH and non-FH hypercholesterolemic children.
MethodsStudy design and patientsThe first phase of this study was a cross-sectional study, and the second phase was a prospective post-TLSC study. From March 2013 to May 2019, 274 children and adolescents aged 4–18 years attending the lipid unit of our University Hospital because of high cholesterol were selected to participate in the “Early Familial Hypercholesterolemia Detection Project” (DECOPIN Project).7 Thirty-six children were not included because of a lack of full clinical information or secondary causes of hypercholesterolemia; therefore, 238 children were included in the cross-sectional phase of the study. Children were classified as FH (n=85) if they had a positive genetic test or LDL-C>160mg/dL and one of the parents had a Dutch Lipid Clinic Network score >8, in the case of no available genetic test result. Children who did not meet the FH criteria were included in the non-FH (n=153) group. None of the children were on lipid-lowering therapy at baseline. The exclusion criteria were chronic renal, hepatic or thyroid disease and type 1 diabetes mellitus, hypercalciuria, eating disorders, autoimmune disease, homozygous FH and other chronic diseases.
All FH children were asked to take part in the prospective phase and all of them accepted. although only 81 have completed the 1 year follow-up and were included in the final analysis. Forty-six non-FH children were also included. In this case, were asked to take part in the prospective phase those children with higher cholesterol (>135mg/dL) and FH siblings willing to participate. After one-year follow-up the main changes in diet and physical activity were reevaluated. Standard lipid profile was also determined. We also communicate the results of a complete 2D-1H-NMR lipid profile that was available at baseline and after one year of follow-up in 97 children (64 FH and 33 non-FH) participating in the prospective phase of the study.
Anthropometry, clinical history and biochemical parametersAnthropometry, demography and clinical data were recorded at basal time (and after the 1-year follow-up in the prospective study). Body mass index (BMI) score was calculated according to the following formula: [(BMI children–BMI 50th percentile of Orbegozo's growth curves)/standard deviation (SD) 50th percentile of Orbegozo's growth curves].28
Standard biochemical analyses were performed at similar times. Blood samples were obtained after overnight fasting. Serum cholesterol and triglyceride levels were evaluated using an enzymatic colorimetric test (CHOD-POD and GPO-POD, respectively), high density lipoprotein cholesterol (HDL-C) was evaluated using a direct enzymatic colorimetric method, and apolipoprotein levels were measured by immunoturbidimetric assays. LDL-C levels were calculated by the Friedewald equation. A portion of the blood sample was stored at −80°C in the BioBanc of our Research Institute.
Full lipoprotein profile (Liposcale Test?)The Liposcale Test? (actualized 2018 version) was used to assess the full lipoprotein profile. As previously reported, this method is based on 2D-1H-NMR.29 This method determines the lipid concentration and particle number for the large, medium and small subclasses of the main lipoprotein classes (VLDL, LDL and HDL) and their size-associated diffusion coefficients. In the actualized version, the LDL lipoprotein class has been linearly calibrated to the LDL particle number according to the standard FDA-approved NMR based on the lipoprotein methodology developed by Otvos and colleagues30 to obtain the best agreement of the absolute LDL particle numbers between the two techniques.31 The variation coefficients for the particle number were between 2% and 4%. The variation coefficients for particle size were lower than 0.3%.
Diet and physical activity assessment and implementation of TLSCThe diet data were collected with a quantitative food frequency questionnaire (FFQ) that included 137 items32 plus alcohol, as validated in the PREDIMED study. The diet data were collected from children and families by a registered nutritionist. The TLSC intervention consisted of nutritionist visits at which children and their families were taught to eat healthy, using simple and healthy culinary techniques and meal plans. They were also motivated to perform physical exercise and avoid alcohol and tobacco consumption. At the end of the clinical visits, a personalized lifestyle recommendation report, consisting in a tailored advice to approach diet to international guidelines according personal deviations, was provided to each participant.
Physical activity (PA) was assessed by the Minnesota leisure-time physical activity questionnaire. According to international guidelines, we quantified the number of hours of physical activity per week.
The Hospital's Ethics Committee approved the study protocol, and this conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Before participation in the study, informed consent was signed by one of the parents.
Statistical analysisThe results are expressed as the mean±SD for normally distributed data, as the median (interquartile range (IQR)) for data that were not normally distributed and as frequencies for categorical data. Kolmogorov–Smirnov tests were used to ensure that the data had a normal distribution. T-tests were used to determine significant differences when the data were normally distributed. Mann–Whitney tests were used to detect significant differences when the data were not normally distributed. The paired T-test were used in normal variables and Wilcoxon signed-rank test in non-parametric variables to know the difference after 1 year of TLSC.
All analyses were performed using the statistical package SPSS 25.0 for Windows (SPSS, IBM®, Chicago, IL). A p-value <0.05 was considered statistically significant in all analyses.
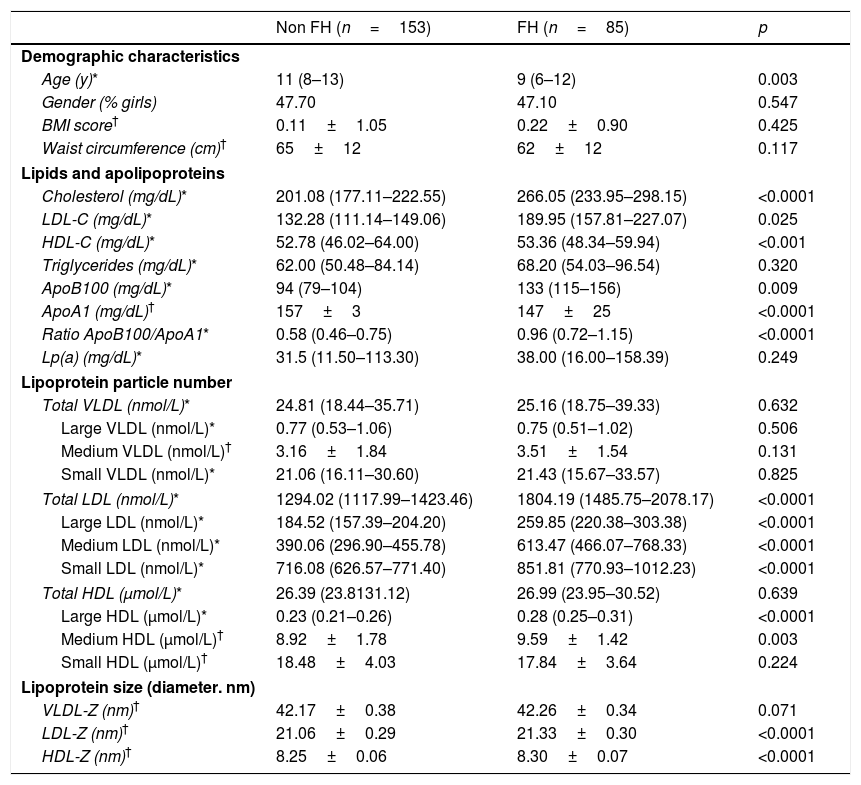
ResultsClinical, biochemical and lipoprotein subclass analysis at basal timeAnthropometry, clinical and lipid profile data from the 238 children are shown in Table 1 according to the diagnostic group. Of these, 85 children had FH (72% positive mutation). The FH children included in the cross-sectional phase were younger than the non-FH children (p=0.003). In the prospective phase, there were no age differences between the groups. As expected, significant differences in total cholesterol (p<0.0001), LDL-C (p=0.025), apolipoprotein B (ApoB) (p=0.009) and apolipoprotein A (ApoA) and its ratio (p<0.0001) were observed. HDL-C was lower in the non-FH group than in the FH group (p<0.0001). No differences in Lp(a) were detected.
Clinical, biochemical and lipoprotein subclass analysis assessed by 2D-1H-NMR of the study subjects at basal time.
| Non FH (n=153) | FH (n=85) | p | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (y)* | 11 (8–13) | 9 (6–12) | 0.003 |
| Gender (% girls) | 47.70 | 47.10 | 0.547 |
| BMI score† | 0.11±1.05 | 0.22±0.90 | 0.425 |
| Waist circumference (cm)† | 65±12 | 62±12 | 0.117 |
| Lipids and apolipoproteins | |||
| Cholesterol (mg/dL)* | 201.08 (177.11–222.55) | 266.05 (233.95–298.15) | <0.0001 |
| LDL-C (mg/dL)* | 132.28 (111.14–149.06) | 189.95 (157.81–227.07) | 0.025 |
| HDL-C (mg/dL)* | 52.78 (46.02–64.00) | 53.36 (48.34–59.94) | <0.001 |
| Triglycerides (mg/dL)* | 62.00 (50.48–84.14) | 68.20 (54.03–96.54) | 0.320 |
| ApoB100 (mg/dL)* | 94 (79–104) | 133 (115–156) | 0.009 |
| ApoA1 (mg/dL)† | 157±3 | 147±25 | <0.0001 |
| Ratio ApoB100/ApoA1* | 0.58 (0.46–0.75) | 0.96 (0.72–1.15) | <0.0001 |
| Lp(a) (mg/dL)* | 31.5 (11.50–113.30) | 38.00 (16.00–158.39) | 0.249 |
| Lipoprotein particle number | |||
| Total VLDL (nmol/L)* | 24.81 (18.44–35.71) | 25.16 (18.75–39.33) | 0.632 |
| Large VLDL (nmol/L)* | 0.77 (0.53–1.06) | 0.75 (0.51–1.02) | 0.506 |
| Medium VLDL (nmol/L)† | 3.16±1.84 | 3.51±1.54 | 0.131 |
| Small VLDL (nmol/L)* | 21.06 (16.11–30.60) | 21.43 (15.67–33.57) | 0.825 |
| Total LDL (nmol/L)* | 1294.02 (1117.99–1423.46) | 1804.19 (1485.75–2078.17) | <0.0001 |
| Large LDL (nmol/L)* | 184.52 (157.39–204.20) | 259.85 (220.38–303.38) | <0.0001 |
| Medium LDL (nmol/L)* | 390.06 (296.90–455.78) | 613.47 (466.07–768.33) | <0.0001 |
| Small LDL (nmol/L)* | 716.08 (626.57–771.40) | 851.81 (770.93–1012.23) | <0.0001 |
| Total HDL (μmol/L)* | 26.39 (23.8131.12) | 26.99 (23.95–30.52) | 0.639 |
| Large HDL (μmol/L)* | 0.23 (0.21–0.26) | 0.28 (0.25–0.31) | <0.0001 |
| Medium HDL (μmol/L)† | 8.92±1.78 | 9.59±1.42 | 0.003 |
| Small HDL (μmol/L)† | 18.48±4.03 | 17.84±3.64 | 0.224 |
| Lipoprotein size (diameter. nm) | |||
| VLDL-Z (nm)† | 42.17±0.38 | 42.26±0.34 | 0.071 |
| LDL-Z (nm)† | 21.06±0.29 | 21.33±0.30 | <0.0001 |
| HDL-Z (nm)† | 8.25±0.06 | 8.30±0.07 | <0.0001 |
FH: familial hypercholesterolemia; BMI: body mass index; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; ApoB100: apolipoprotein B100; ApoA1: apolipoprotein A1; Lp(a): lipoprotein (a); Z: size.
The data are presented as the mean±standard deviation for normally distributed data. median (Q1–Q3) for non-normally distributed data or percentage for categorical variables.
FH children had significantly higher absolute concentrations of total, large, medium and small LDL subclasses (p<0.0001) than the non-FH children. The number of small LDL particles was approximately 20% higher in FH children than in non-FH children. The mean LDL size was slightly larger in FH children than in non-FH children because of a higher proportion of medium-sized LDL at baseline.
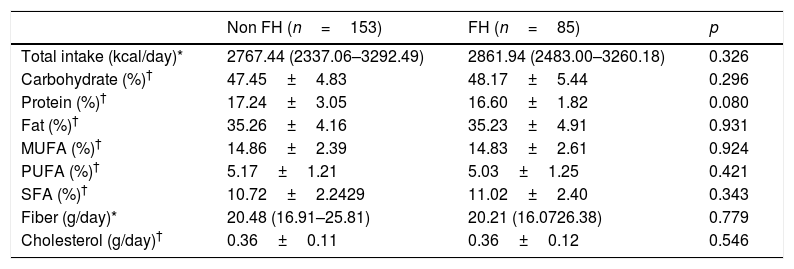
Diet composition and intake of macronutrients at basal timeThe intake of macronutrients is shown in Table 2. At basal time, we did not find differences in diet composition between groups. Both groups ingested a high amount of calories (median values for FH=2767kcal and non-FH=2861kcal). The consumption of protein, saturated fatty acid (SFA) and cholesterol was well above the general recommendations. The calorie intake delivered by SFA was 11%, which is much higher than the recommended 7–8%. The amount of fiber was approximately 20g/day, which was closer to the lower recommended limits.
Nutritional information of the daily intake of children evaluated from a food frequency questionnaire (FFQ) at basal time (N=238).
| Non FH (n=153) | FH (n=85) | p | |
|---|---|---|---|
| Total intake (kcal/day)* | 2767.44 (2337.06–3292.49) | 2861.94 (2483.00–3260.18) | 0.326 |
| Carbohydrate (%)† | 47.45±4.83 | 48.17±5.44 | 0.296 |
| Protein (%)† | 17.24±3.05 | 16.60±1.82 | 0.080 |
| Fat (%)† | 35.26±4.16 | 35.23±4.91 | 0.931 |
| MUFA (%)† | 14.86±2.39 | 14.83±2.61 | 0.924 |
| PUFA (%)† | 5.17±1.21 | 5.03±1.25 | 0.421 |
| SFA (%)† | 10.72±2.2429 | 11.02±2.40 | 0.343 |
| Fiber (g/day)* | 20.48 (16.91–25.81) | 20.21 (16.0726.38) | 0.779 |
| Cholesterol (g/day)† | 0.36±0.11 | 0.36±0.12 | 0.546 |
FH: familial hypercholesterolemia; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; SFA: saturated fatty acid.
The data are presented as the mean±standard deviation for normally distributed data. median (Q1–Q3) for non-normally distributed data or percentage for categorical variables.
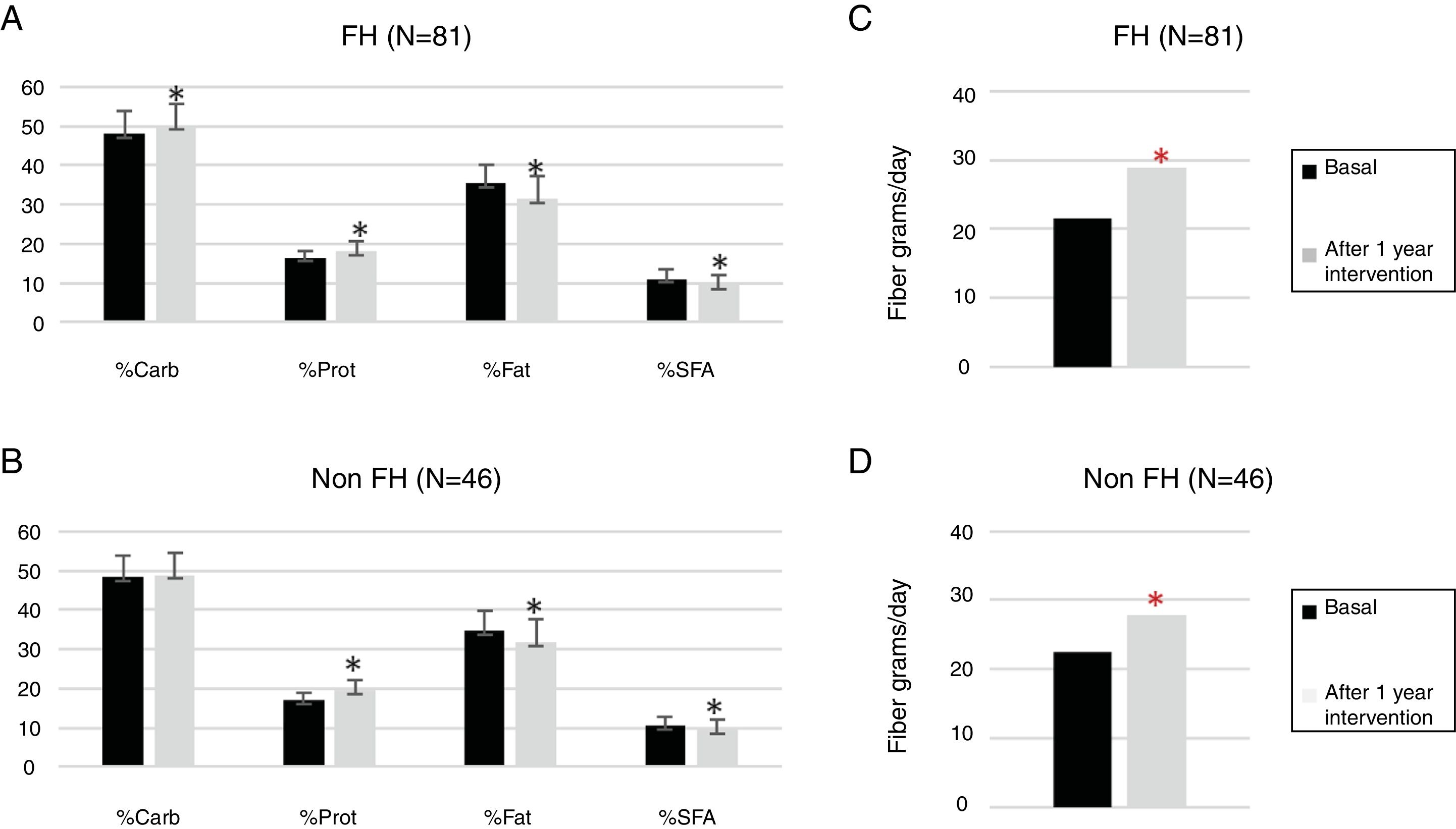
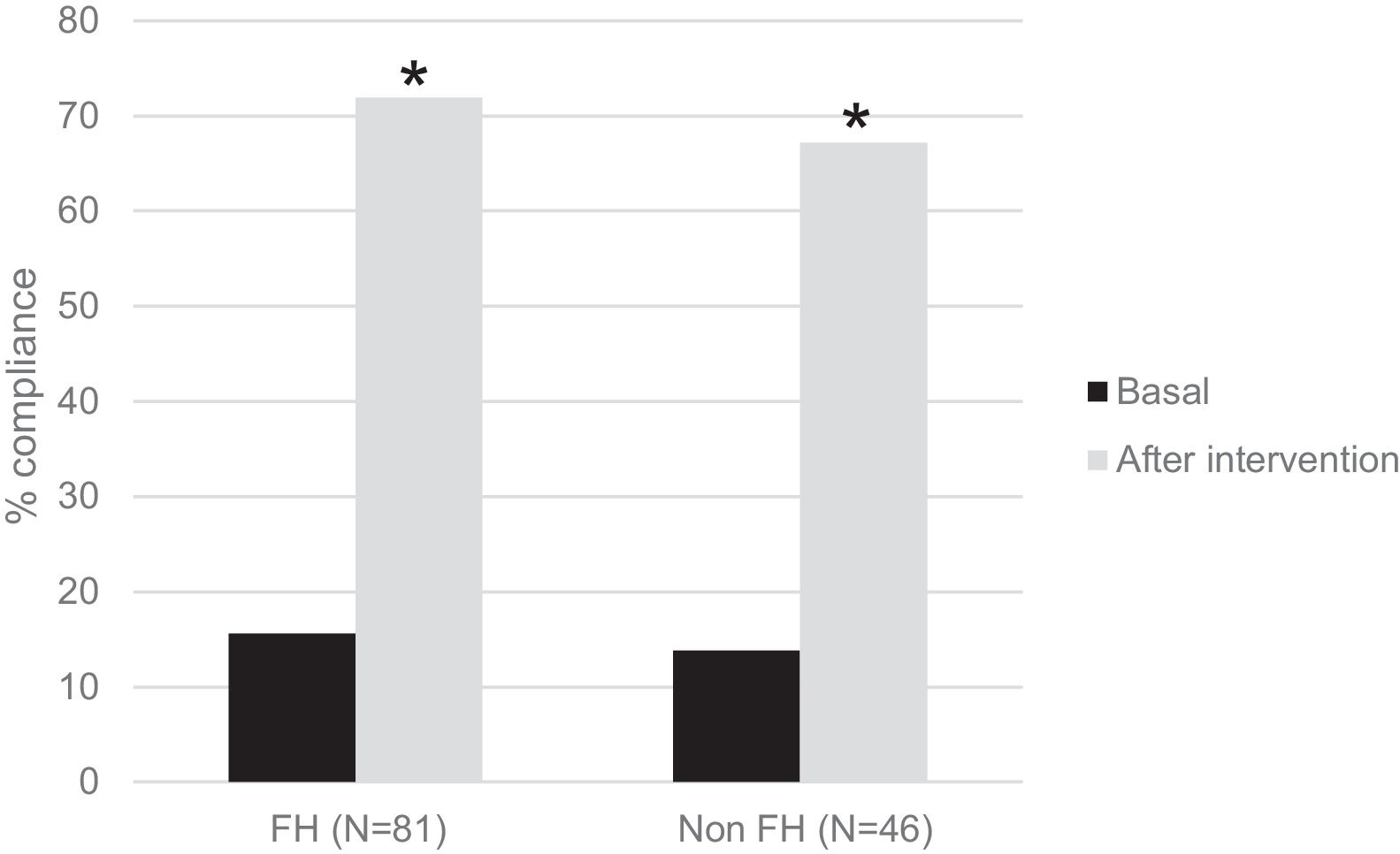
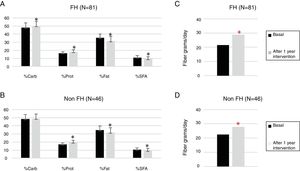
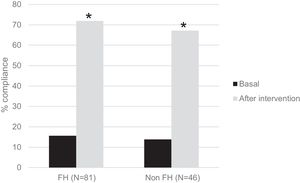
One hundred twenty-seven children participated in the prospective phase (FH n=81 and non-FH n=46). After one year of TLSC, both diet composition and physical activity changed significantly in a positive way (Figs. 1 and 2).
Changes in the daily intake evaluated from a food frequency questionnaire (FFQ) at basal time and after intervention.
FH: familial hypercholesterolemia; Carb: carbohydrate; Prot, protein; SFA, saturated fatty acid.
p Values were obtained by paired sample T-test.
*The difference after 1 year of TLSC is statistically significant (p<0.05).
The percentages of calories due to total fat (FH p<0.0001 and non-FH p=0.002) and saturated fat (FH p<0.0001 and non-FH p=0.020) were significantly reduced. The amount of fiber increased significantly by approximately 10% in both groups (FH p<0.0001 and non-FH p=0.003).
Interestingly, in Fig. 2, we show the percentage of children who performed 1h/day of PA before and after the intervention. There were significant increases of 4,6-fold in the FH group and of 4,8-fold in the non-FH group (p<0.0001 in both cases).
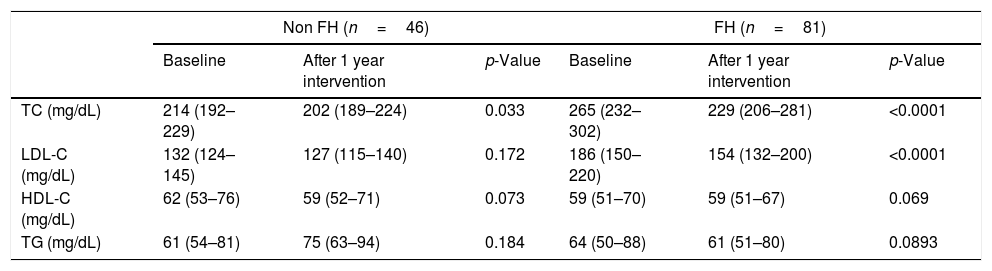
Lipid profile after one year of the TLSC interventionThe standard lipid profile, prospectively determined in 127 children, showed a decrease in total and LDL cholesterol in both hypercholesterolemic groups, although the LDL-C reduction was only statistically significant in FH children (FH p<0.0001 and non-FH p=0.172) (Table 3). The magnitude of change was larger in the FH group than in the non-FH group (Table 3). There were no significant changes in HDL-C and triglycerides.
Lipid profile after 1-year intervention (N=127).
| Non FH (n=46) | FH (n=81) | |||||
|---|---|---|---|---|---|---|
| Baseline | After 1 year intervention | p-Value | Baseline | After 1 year intervention | p-Value | |
| TC (mg/dL) | 214 (192–229) | 202 (189–224) | 0.033 | 265 (232–302) | 229 (206–281) | <0.0001 |
| LDL-C (mg/dL) | 132 (124–145) | 127 (115–140) | 0.172 | 186 (150–220) | 154 (132–200) | <0.0001 |
| HDL-C (mg/dL) | 62 (53–76) | 59 (52–71) | 0.073 | 59 (51–70) | 59 (51–67) | 0.069 |
| TG (mg/dL) | 61 (54–81) | 75 (63–94) | 0.184 | 64 (50–88) | 61 (51–80) | 0.0893 |
FH: familial hypercholesterolemia; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: blood triglycerides.
p Values were obtained by paired sample T-test for normal variables and Wilcoxon signed-rank test for non-parametric variables.
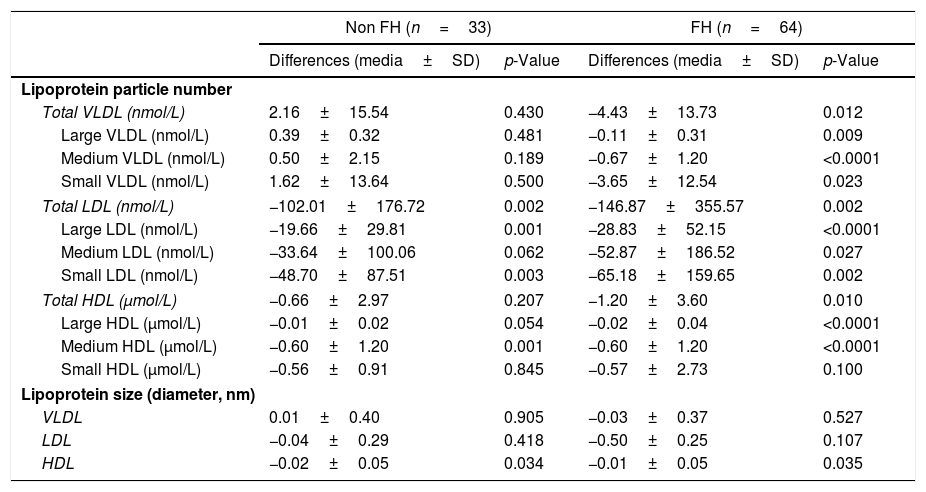
The lipid profile assessed by 2D-1H-NMR determined prospectively in 97 children, Table 4, showed a significant decrease in total LDL particles in both groups (FH and non-FH p=0.002), while maintaining the same proportion of particles among subclasses. The absolute number of small LDL particles was significantly reduced in both cases (FH p=0.002 and non-FH p=0.003). The mean LDL size was not changed in any group. VLDL and HDL total particle number was also reduced in both groups although the difference was only significant in the FH group, probably reflecting the sample size differences.
Changes in lipoprotein subclass analysis assessed by 2D-1H-NMR after one year of intervention in lifestyle changes (N=97).
| Non FH (n=33) | FH (n=64) | |||
|---|---|---|---|---|
| Differences (media±SD) | p-Value | Differences (media±SD) | p-Value | |
| Lipoprotein particle number | ||||
| Total VLDL (nmol/L) | 2.16±15.54 | 0.430 | −4.43±13.73 | 0.012 |
| Large VLDL (nmol/L) | 0.39±0.32 | 0.481 | −0.11±0.31 | 0.009 |
| Medium VLDL (nmol/L) | 0.50±2.15 | 0.189 | −0.67±1.20 | <0.0001 |
| Small VLDL (nmol/L) | 1.62±13.64 | 0.500 | −3.65±12.54 | 0.023 |
| Total LDL (nmol/L) | −102.01±176.72 | 0.002 | −146.87±355.57 | 0.002 |
| Large LDL (nmol/L) | −19.66±29.81 | 0.001 | −28.83±52.15 | <0.0001 |
| Medium LDL (nmol/L) | −33.64±100.06 | 0.062 | −52.87±186.52 | 0.027 |
| Small LDL (nmol/L) | −48.70±87.51 | 0.003 | −65.18±159.65 | 0.002 |
| Total HDL (μmol/L) | −0.66±2.97 | 0.207 | −1.20±3.60 | 0.010 |
| Large HDL (μmol/L) | −0.01±0.02 | 0.054 | −0.02±0.04 | <0.0001 |
| Medium HDL (μmol/L) | −0.60±1.20 | 0.001 | −0.60±1.20 | <0.0001 |
| Small HDL (μmol/L) | −0.56±0.91 | 0.845 | −0.57±2.73 | 0.100 |
| Lipoprotein size (diameter, nm) | ||||
| VLDL | 0.01±0.40 | 0.905 | −0.03±0.37 | 0.527 |
| LDL | −0.04±0.29 | 0.418 | −0.50±0.25 | 0.107 |
| HDL | −0.02±0.05 | 0.034 | −0.01±0.05 | 0.035 |
FH: familial hypercholesterolemia; VLDL: very low-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; SD: standard deviation.
p Values were obtained by paired sample T-test for normal variables and Wilcoxon signed-rank test is a non-parametric variables.
In this study, we report that TLSC implemented in children with genetically driven hypercholesterolemia produced a global benefit on diet and PA, leading to lipoprotein profile improvement and therefore cardiovascular risk reduction.33–34
Lifestyle changes are the first option in the treatment of FH children. We have observed that healthy lifestyle recommendations lead to a beneficial change in both diet composition and the amount of PA.1,2,8,35 In our study, the diet of both FH and non-FH children improved. The percentages of fats and SFA were reduced in both groups. SFA intake is associated with higher total cholesterol and LDL-C,8 and controlling cholesterol and SFA intake has no negative impact on the growth and development of children.36 The percentage of protein increased slightly in both groups, but the type of food carrying them changed positively (data not shown). The fiber content of the children's diet increased significantly in the two groups. An increase in dietary fiber has been associated to a lipid profile improvement.17,20
PA was also improved. Regular and sustained physical activity protects against cardiovascular disease with a dose-response relationship. It is recommended that children perform 60min of moderate to vigorous PA every day.37
Another aspect to be highlighted is the importance of early TLSC. Establishing lifestyle changes at early ages is more effective, better assimilated and better maintained over time than those established later in life.1,8 Early prevention of atherosclerotic disease should begin in childhood with lifestyle education.34
Interestingly, TLSC were associated with positive modifications of the lipid profile, not just on standard parameters but also on total and small LDL particle numbers.27,34 Notably, these changes were more significant in FH children, probably because the differences in the sample size and that they started from higher basal values, but a greater empowerment and adherence, because children and their families were more aware of the disease could also play a role. Interesting, VLDL and HDL were also reduced probably reflecting the impact on global fat burden. The reduction on HDL particles, without a HDL-C lowering, could reflect cholesterol redistribution among bigger particles.
Despite the genetic origin of hypercholesterolemia, TLSC have a significant impact on lipid parameters. According to the Cholesterol Treatment Trialist Collaboration meta-analyses, based on statin randomized control trials, each 1 mmol/L of LDL-C reduction is associated with a 22% relative cardiovascular risk reduction.38 Data from observational studies and mainly from Mendelian randomized studies show a greater benefit when the LDL-C reduction starts earlier.39 Accordingly, our results suggest a higher impact on cardiovascular risk in these groups of children over time. Moreover, TLSC are associated not only with LDL-C reduction but also with total and, interestingly, small LDL particles, which should induce an incremental overall benefit.
The main limitations of this study are, first, the limited sample size. However, this is one of the larger cohorts reported prospectively. The sample sizes of the crossectional study, the global prospective and the 2D-1H-NMR prospective study are different, according data availability. However, the demographic data of groups was not significantly different.
In our cohort, we have adolescents, and in this period of development, LDL-C levels are changing, which could jeopardize the real effect of TLSC.40 Finally, diet assessments are always inaccurate, even though we used validated questionnaires for the Spanish population, and the data acquisition was performed by the same nutritionist.
In conclusion, educational strategies to implement TLSC in children lead to empowerment, increased adherence and overall metabolic improvement in hypercholesterolemic children, including those with FH. Early intervention should result in an important impact on future cardiovascular prognosis.
Financial supportThis study was funded by 2 projects:
- -
“Manuel de Oya” nutritional research grant. This grant was awarded by the “Sociedad Española de Arteriosclerosis” and the “Fundación Española de Arteriosclerosis” in 2017 in the XXX Congress of Cádiz (title of the project: “Impact of lifestyle changes on lipidomics and the lipoprotein profile evaluated by nuclear magnetic resonance (2D-1H NMR) in children affected with Familial Hypercholesterolemia”).
- -
The “Marato de TV3” (title of the project: Preventing Premature Coronary Heart Disease in Catalonia by Expanding Familial Hypercholesterolemia Diagnosis); grant number 20152430.
Cèlia Rodríguez-Borjabad: Rodríguez-Borjabad contributed to the collection, analysis and interpretation of the data and drafted the initial manuscript.
Ana Irene Malo: Dr. Malo contributed to the collection and critically reviewed the manuscript.
Daiana Ibarretxe: Dr. Ibarretxe conceptualized and designed the study, contributed to the collection, analysis and interpretation of the data and critically reviewed the manuscript.
Josefa Girona: Dr. Girona contributed to the data analysis and critically reviewed the manuscript.
Merche Heras: Mrs contributed to analysis of blood samples
Raimon Ferre: Dr. Ferré performed carotid sonography and critically reviewed the manuscript.
Albert Feliu: Dr. Feliu conceptualized and designed the study, contributed to the recruitment of patients and to the initial review of eligibility according to the inclusion criteria and critically reviewed the manuscript.
Maria Salvadó: Dr. Salvadó contributed to the recruitment of patients.
Anna Varela: Mrs. Varela was the nurse in charge of collecting the blood samples.
Núria Amigó: Dr. Amigó carried out a study of lipoproteins by NMR.
Luís Masana: Dr. Masana conceptualized and designed the study, drafted the initial manuscript, supervised the data collection and approved the final manuscript as submitted. Núria Amigó: contributed to the NMR analysis and critically reviewed the manuscript.
Núria Plana: Dr. Plana conceptualized and designed the study, contributed to the data collection, supervised the data collection, drafted the initial manuscript and approved the final manuscript as submitted.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflicts of interestDaiana Ibarretxe: lecture stipends from Sanofi, MSD and Esteve. Núria Plana: lecture stipends from Amgen, MSD, Ferrer and Alexion. Luís Masana: lecture stipends and advisory fees from Amgen, Sanofi and MSD. Núria Amigo: shareholder of Biosfer Teslab. None of these conflicts of interest are related to the contents of the present manuscript. The other authors have indicated that they have no potential conflicts of interest to disclose.
We sincerely thank all the participants and their families for participating in this project.
We also thank the entire Primary Care Pediatrician Group (Decopin Group) for assistance in the recruitment of participants and the FH families for their valuable contribution and willingness to participate.
Aguado Fèlix (CAP Marià Fortuny, Reus), Amigó Elisabet (Hospital Sant Pau i Santa Tecla, Tarragona), Andrés Patricia (CAP de Riudoms, Reus), Barrio Mercedes (CAP del Morell, Morell), Bilbao José Ángel (ABS de Riudoms, Riudoms), Bosch Montserrat (CAP Salou, Salou), Cabedo Jose Luis (CAP Mariá Fortuny, Reus), Calvo Josefa (Hospital Sant Pau i Santa Tecla,Tarragona), Campillo Carmen (CAP Torreforta-La Granja,Tarragona), Caselles Alejandra (Cap Riudoms, Reus), Castejón Enma (ABS Selva del Camp, La Selva del Camp), Castillejo Gemma (Hospital Universitari Sant Joan,Reus), Castro Maria (Hospital Sant Pau i Santa Tecla,Tarragona), Cliville Rosa (CAP Sant Pere, Reus), De Gotardo Enrique (Hospital Sant Pau i Santa Tecla,Tarragona), De La Hoz Rebeca (CAP Roquetes, Roquetes de Mar), Domènech Vanesa (CAP Amposta, Amposta), Domínguez Dolores (CAP Muralla,Tarragona), Escolà Maria (CAP Roquetes, Roquetes), Fernández Marta (Hospital Universitari Joan XXIII,Tarragona), García Joan (CAP de Sant Pere, Reus), Girona Raquel (Consultori local El Pla de Santa Maria, Pla de Santa Maria), Gispi Sílvia (CAP Jaume I, Tarragona), Guàrdia Jara (CAP Sant Pere, Reus) Guijarro Eugenio (CAP Bonavista, Tarragona), Gutierrez MªAntonia (CAP Constantí, Tarragona), Iglesias Dolores (CAP Torreforta-La Granja, Tarragona), Jiménez Marta (Hospital Sant Pau i Santa Tecla, Tarragona), Luque Verónica (Hospital Universitari Joan XXIII, Tarragona), Machado Pilar (CAP Torreforta-La Granja, Tarragona), Maixé Jordi (Hospital Sant Pau i Santa Tecla, Tarragona), Mallafré Marta (Hospital Lleuger Antoni de Gimbernat, Cambrils), Martin Ramona (CAP Mª Fortuny, Reus), Jiménez Milagros (CAP Horts de Miro, Reus), Monne Raquel (Hospital Universitari Joan XXIII,Tarragona), Morales Raquel (CAP Sant Pere 1, Reus), Morillo Susana (CAP Llibertat, Reus), Naranjo Àngels (CAP Espluga de Francolí, L’Espluga de Francolí), Pérez Cristina (CAP Llibertat, Reus), Pérez MªTeresa (CAP Sant Pere, Reus), Planelles Montserrat (CAP Mª Fortuny, Reus), Querol Cecilia (CAP de Sant Pere, Reus), Rabadà MªJosé (CAP Selva del Camp, La Selva del Camp), Remedi Ayelen (Hospital Comarcal Móra d’Ebre, Mòra d’Ebre), Riquelme Carmen (Hospital Sant Pau i Santa Tecla,Tarragona), Rodríguez Neus (Hospital Verge de la Cinta,Tortosa), Rosell Laura (CAP Llibertat, Reus), Roset Laura, Salsas Jaume Miquel (CAP Santa Bárbara, Santa Bárbara), Salvadó Maria (Cap Sant Pere, Reus), Salvador Olga (CAP Llibertat, Reus), Santos Alicia (Hospital Universitari Joan XXIII, Tarragona), Segura Sandra (CAP Montroig del Camp, Montroig del Camp), Subirana Gloria (CAP Rambla Nova, Tarragona), Tarrades Pilar (Hospital Pius, Valls), Vendrell Montserrat (ABS Vandellós i Hospitalet del Infant, L’Hospitalet de L’Infant), Vilella Mireia (CAP Rambla Nova, Tarragona) and Zabala Eduardo (CAP Sant Pere 1, Reus).