Dysfunction of perivascular adipose tissue of mesenteric bed participates in the pathophysiology of high blood pressure linked to metabolic syndrome. Thus, it might consider a new therapeutic objective to take account in cardiovascular and metabolic diseases. Besides its antihypertensive effect, there is a growing interest on the pleiotropic actions of losartan, an angiotensin II type 1 (AT1) receptor antagonist. The aim of the study was to analyze the actions of losartan treatment on adiposity index and prostanoids release from mesenteric vascular bed and its relationship with blood pressure as well as homeostasis model of assessment of insulin resistance (HOMA-IR) in Sprague-Dawley rats under a high-fat (HF) diet for 8 weeks. Four groups were used: control (C), HF diet (HF, 50%, w/w bovine fat), losartan-treated (CL8, 30mg/kg/body weight/day in the drinking water) and losartan-treated HF diet (HFL, both treatments). A high-fat diet incremented systolic blood pressure, HOMA-IR, adiposity of mesenteric vascular bed and the release of vasoconstrictor prostanoids such as thromboxane (TX) B2 and prostaglandin (PG) F2α as well as PGE2, an inflammatory prostanoid in a context of insulin resistance and hypertension. We found a positive correlation between adiposity index and systolic blood pressure. Also, both parameters are positive correlated with the HOMA IR index. Moreover, we also found that these prostanoids release correlate with systolic blood pressure as well as with mesenteric vascular bed adiposity index. Losartan treatment prevented all these alterations and normalized the PGI2/TXA2 ratio in high-fat fed rats. We conclude that losartan may play beneficial actions on perivascular adipose tissue alterations and endothelial dysfunction through restoration of normal balance of vasoactive substances in this model.
La disfunción del tejido adiposo perivascular del lecho mesentérico posee una participación en la fisiopatología de la hipertensión arterial relacionada con el síndrome metabólico. Por lo tanto, podría considerarse como un nuevo blanco terapéutico en las enfermedades cardiovasculares y metabólicas. Además de su efecto antihipertensivo, existe un interés creciente en las acciones pleiotrópicas de losartán, antagonista del receptor de angiotensina II. El objetivo del estudio fue analizar las acciones de losartán sobre el índice de adiposidad y la liberación de prostanoides del lecho vascular mesentérico y su relación con la presión arterial, así como en el índice HOMA-IR (modelo de evaluación homeostático de la resistencia a la insulina) en ratas con dieta alta en grasas. Observamos que la dieta alta en grasas incrementó la adiposidad del lecho vascular mesentérico y la liberación de prostanoides vasoconstrictores como tromboxano (TX) B2 y prostaglandina (PG) F2α, así como la PGE2, un prostanoide inflamatorio en el contexto de resistencia a la insulina e hipertensión. También encontramos una correlación positiva entre el índice de adiposidad y la presión arterial sistólica y ambos parámetros se correlacionan positivamente con el índice HOMA IR. Adicionalmente observamos que la liberación de estos prostanoides se correlaciona con la presión arterial sistólica, así como con el índice de adiposidad del lecho vascular mesentérico. El tratamiento con losartán previno todas estas alteraciones y normalizó la relación PGI2/TXA2 en ratas alimentadas con una dieta alta en grasa. Concluimos entonces que losartán puede ejercer acciones beneficiosas sobre las alteraciones del tejido adiposo perivascular y la disfunción endotelial a través de la restauración del equilibrio normal de sustancias vasoactivas en este modelo experimental.
Dysfunction of perivascular adipose tissue (PVAT) plays a role in the pathogenesis of hypertension associated to metabolic syndrome and cardiovascular diseases.1–4 In this way, ectopic fat deposition in PVAT could be relevant to trigger insulin resistance. Also, a local renin–angiotensin–aldosterone system (RAAS) in adipose tissue is involved in metabolic syndrome development.5–7 In this context, pleiotropic effects of drugs used for the treatment of hypertension associated to metabolic diseases, such as losartan, an angiotensin II type 1 (AT1) receptor blocker, still generate great interest.8
PVAT has an active contribution in the regulation of vascular function. The physiological anti-contractile and anti-inflammatory effects of PVAT are diminished in hypertension.9,10 From its first mention by Leonardo da Vinci, mesenteric vascular bed remained almost without clinical relevance.11 It is formed by resistance arteries surrounded by PVAT, mainly of white visceral adipose tissue.12,13 On the other hand, PVAT of the mesenteric arteries has a local RAS with high density of AT1 receptors.14,15
In addition, mesenteric vascular bed is a source of prostanoids derived from the action of cyclooxygenases that includes prostaglandins (PGs) and thromboxanes (TXs) which participate in vascular tone regulation. These agents are synthesized and released from endothelial and smooth muscle cells as well as in adipose tissue of mesenteric vascular bed.16 Therefore dysfunctional PVAT on those vessels as a result of a high-fat diet could be a possible link between metabolic diseases, high blood pressure and vascular complications. Concomitantly, we have previously demonstrated alterations in prostanoids release in experimental models of metabolic syndrome.17,18 However, little is known about the role of RAS in the development of PVAT on mesenteric vascular bed. Thus, the aim of this study was to analyze the effects of losartan on the adiposity and prostanoids release from mesenteric vascular bed and its relationship with blood pressure and insulin resistance in rats under a high-fat diet.
Material and methodsEthical approvalThe experimental protocol was previously approved by the local Comité Institucional para el Cuidado y Uso de Animales de Laboratorio (CICUAL; Facultad de Farmacia y Bioquímica; Universidad de Buenos Aires; Resolution N° 1881-19999) according to the International Principles for Research on Animals. All the animals were housed with a 12h light/dark cycle, controlled temperature (22±2°C) and adequate humidity.
Animal's protocol and dietTwenty-four male Sprague-Dawley rats (weighing 180–210g at the beginning) were studied for 9 weeks. They were randomly divided into four groups (n=6 each group): control group (C) were fed standard rodent diet (SD, 3.3kcal/g; with 2% fiber, 3% fat, 6% minerals, 20% proteins and 69% starch and vitamins supplements; Commercial Rodents Purina Chow, Asociación Cooperativas Argentinas SRL, Buenos Aires, Argentina) and water to drink; high-fat diet group (HF), which received 50% (w/w) bovine fat (BF, 9kcal/g; 99% total fat: 77% saturated fat and 19% trans-fat, and necessary amounts of carbohydrates, protein, fiber, sodium, vitamins and minerals supplements; Quickfood S.A. Provincia de Santa Fe, Argentina) added to 50% (w/w) SD and water to drink; losartan-control group (CL), which received losartan (30mg/kg/bodyweight/day, highest available commercial grade was purchased from Droguería Saporiti S.A.C.I.F.I.A, Buenos Aires, Argentina) dissolved in the drinking water and fed SD; and losartan-high-fat diet group (HFL), which received losartan (same dose) in the water and 50% (w/w) BF added to 50% (w/w) SD. All animals were given free access to water and food, ad libitum. Body weight, food and water intake were monitored throughout the entire period in all experimental groups. Dietary and pharmacological treatments began at the same time. The dose of losartan was chosen according to previous studies.19–21
Blood analysisAt the end of the study period, rats were fasted for 5h, weighed and blood samples were collected from the retro-ocular sinus under light anesthesia.22,23 Plasma glucose levels were measured by glucose meter (Roche Accu-Chek®, Germany); plasma triglyceride levels were evaluated using commercial kits (enzymatic methods, TG Color Wiener Laboratories, S.A.I.C, Rosario, Argentina) and insulin levels by rat/mouse insulin ELISA kit (Merck Millipore, USA). The following equation was used to determine the homeostasis model of assessment of insulin resistance (HOMA-IR)=glucose (mM)×insulin (mIUl−)/22.5.24
Adiposity index and blood pressureRats were weighed prior to dietary and pharmacological manipulation and at the end of the study. The entire mesenteric vascular bed that includes mesenteric blood vessels with PVAT was dissected and weighed from each animal. We calculated the mesenteric vascular bed adiposity index as its weight/body weight×100. For two weeks prior to the end of the experimental period, all animals were trained to the procedure of systolic blood pressure (SBP) measurement, which was performed by indirect method of tail cuff plethysmography (Tektronic Inc., Portland, OR, USA).
Prostanoid release measurementThe mesenteric vascular bed dissected tissue was embedded with Krebs solution (mM; KCl 4.7, NaCl 118.0, NaH2PO4 1.0, MgSO4 1.2, CaCl2 2.6, NaHCO3 25.0, glucose 11.1), and incubated during 60min at 37°C. Then, the media was acidified (pH 3.5) with 1M formic acid and extracted with chloroform to measure prostanoid release. Dried extracted chloroform samples were suspended in the mobile phase and injected into the Reversed-phase HPLC system (BBS Hypersil C18, Thermo Electron Co., Bellefonte, PA, USA). The following prostanoids standards were run together with the samples: 6-keto PGF1α (stable metabolite of PGI2 or prostacyclin), PGE2, PGF2α and TXB2 (stable metabolite of TXA2) (Sigma Chemical Co., Saint Louis, MO, USA). The results were expressed as nanograms of prostanoid per milligram of wet tissue weight.
StatisticsStatistical analysis was performed using InfoStat software program, version 2018 (Córdoba, Argentina), by means of two-way ANOVA and Tukey's post hoc test. For correlation analysis, Pearson's correlation coefficients (r) of the data points from experimental rats were calculated by linear regression. A P<0.05 was considered statistically significant. All results are expressed as the mean±SEM.
ResultsCaloric intake, body weight and metabolic parametersAs shown in Table 1, caloric intake was significantly higher in HF compared to C rats (P<0.01). Losartan did not alter total calories intake in CL and HFL groups compared to C and HF, respectively. At the end of period study, high-fat diet produced an increase in body weight in HF compared to C rats (P<0.05). HF fed rat exhibited increased triglyceridaemia, glycaemia and insulinaemia as well as the HOMA-IR with respect to C group (P<0.01; Table 1). These results indicate that insulin resistance model in metabolic syndrome was effectively induce by high-fat diet. Losartan treatment ameliorated all these alterations in HFL compared to HF (P<0.01; Table 1).
Caloric intake, body weight and metabolic parameters.
| C | CL | HF | HFL | |
|---|---|---|---|---|
| Caloric intake (Kcal/day) | 73.7±1.2 | 74.9±1.2 | 103.8±2.5* | 102.7±1.6* |
| Body weight (g) | 424.4±19.2 | 421.0±22.5 | 477.1±8.5# | 441.5±16.1 |
| Triglycerides (mg/dl) | 71±3 | 73±6 | 166±9* | 88±4*** |
| Glycemia (mg/dl) | 108±4 | 114±6 | 148±4* | 129±3*** |
| Insulinemia (ng/ml) | 1.2±0.1 | 1.0±0.1 | 4.9±0.5* | 2.3±0.2**** |
| HOMA-IR | 0.11±0.01 | 0.09±0.01 | 0.57±0.06* | 0.23±0.05**,# |
Results are expressed as mean±SEM. Control (C); high-fat diet (HF); losartan-control (CL); losartan-high-fat diet (HFL).
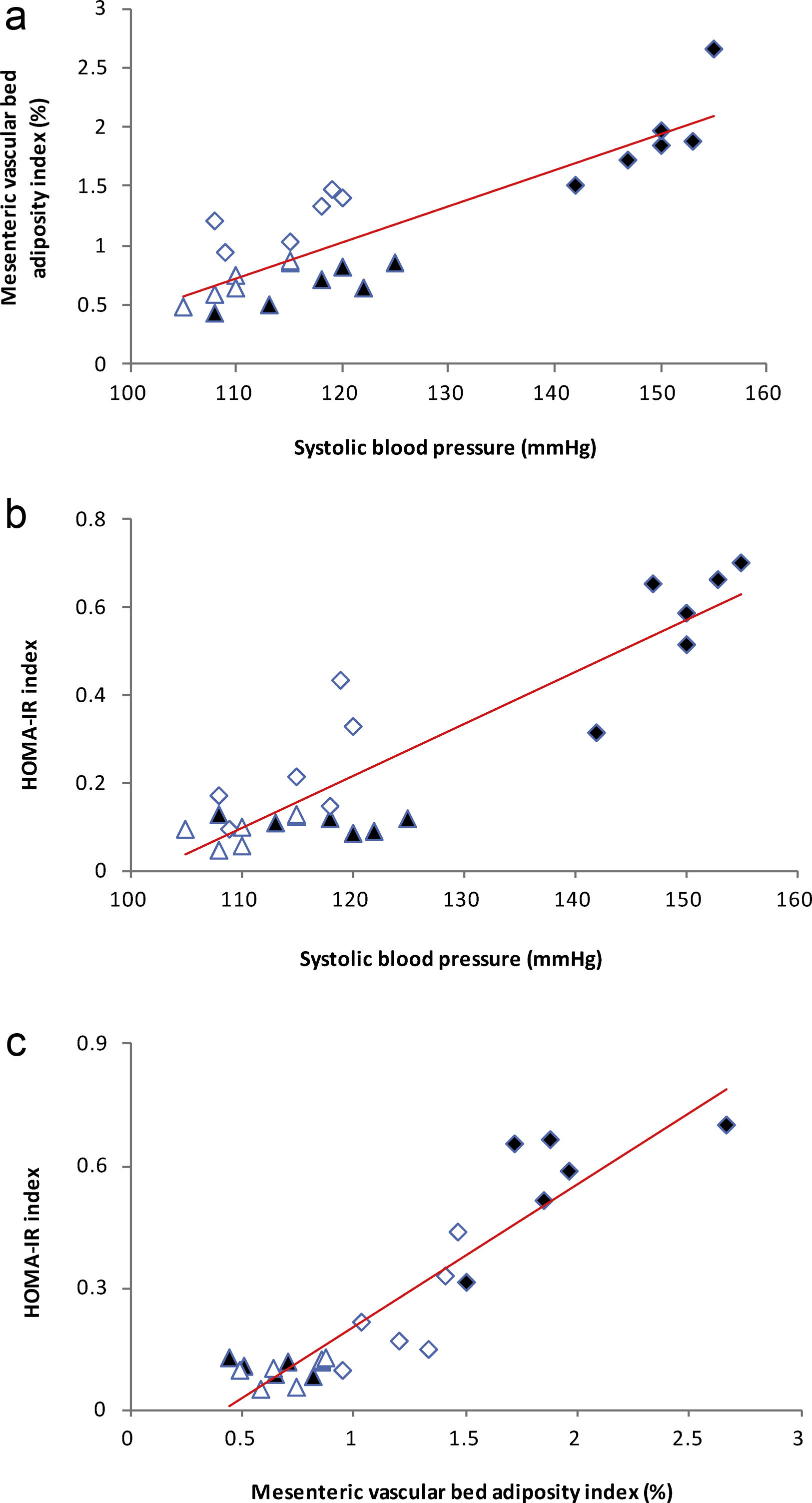
High-fat diet produced increments on mesenteric vascular bed adiposity index and SBP in HF rats compared to C (P<0.01; Table 2). Losartan treatment not only prevented SBP rise as expected (P<0.01; Table 2), but also prevented the increase of mesenteric vascular bed adiposity index in HFL compared to HF (P<0.01; Table 2). A positive correlation was found between adiposity index of mesenteric vascular bed and SBP (r=0.87, R2=0.80, P<0.01; Fig. 1a). Also, significant correlations were found between SBP and HOMA-IR (r=0.90, R2=0.80, P<0.01; Fig. 1b) as well as between mesenteric vascular bed adiposity index and HOMA-IR (r=0.93, R2=0.86, P<0.01; Fig. 1c).
Mesenteric vascular bed adiposity and systolic blood pressure.
| Mesenteric vascular bed adiposity index (%) | Systolic blood pressure (mm Hg) | |
|---|---|---|
| C | 0.7±0.1 | 118±2 |
| CL | 0.7±0.1 | 111±2## |
| HF | 2.0±0.1* | 150±2* |
| HFL | 1.2±0.1**** | 115±2** |
Results are expressed as mean±SEM. Control (C); high-fat diet (HF); losartan-control (CL); losartan-high-fat diet (HFL).
(a) Linear regression of systolic blood pressure against mesenteric vascular bed adiposity index: Control (
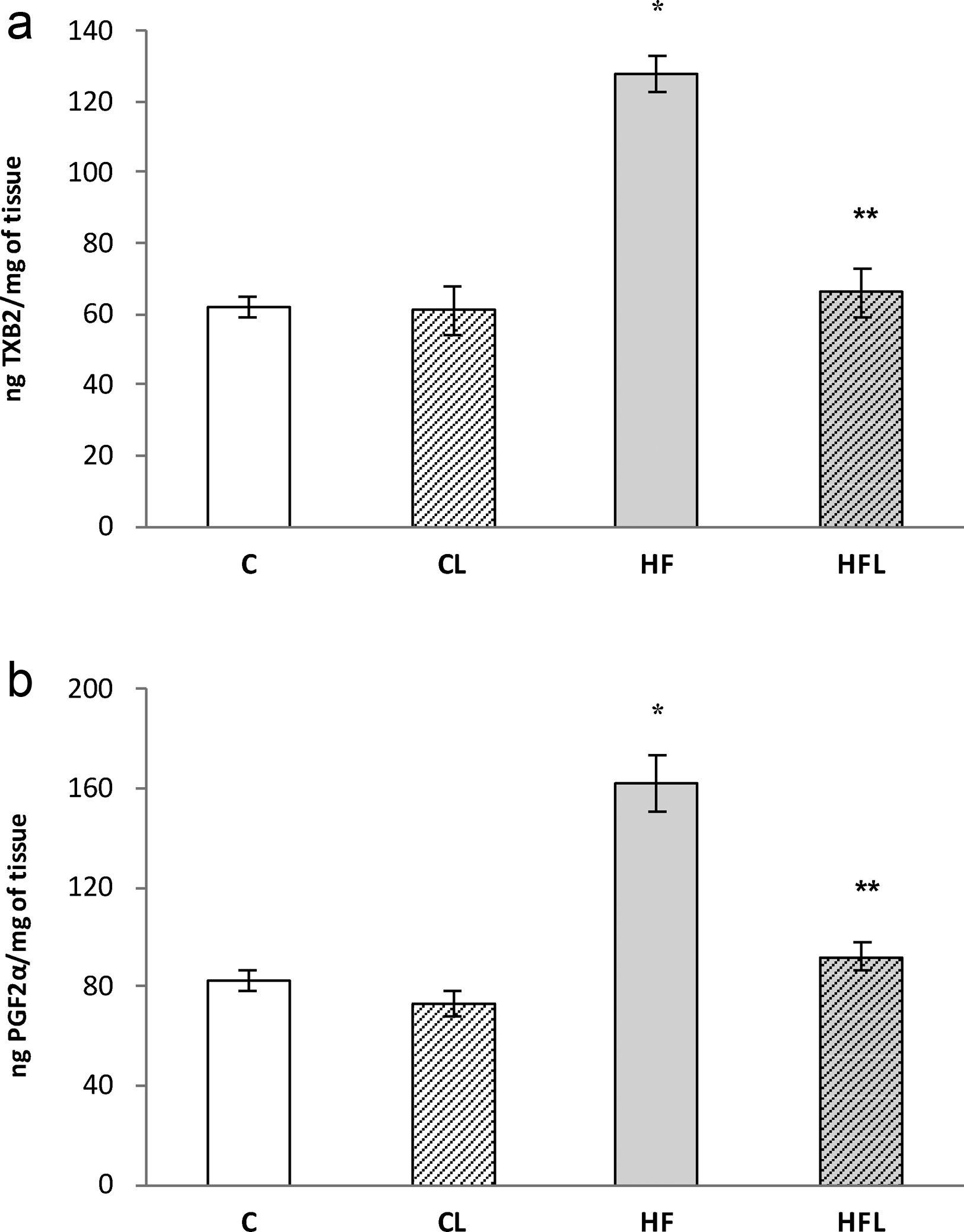
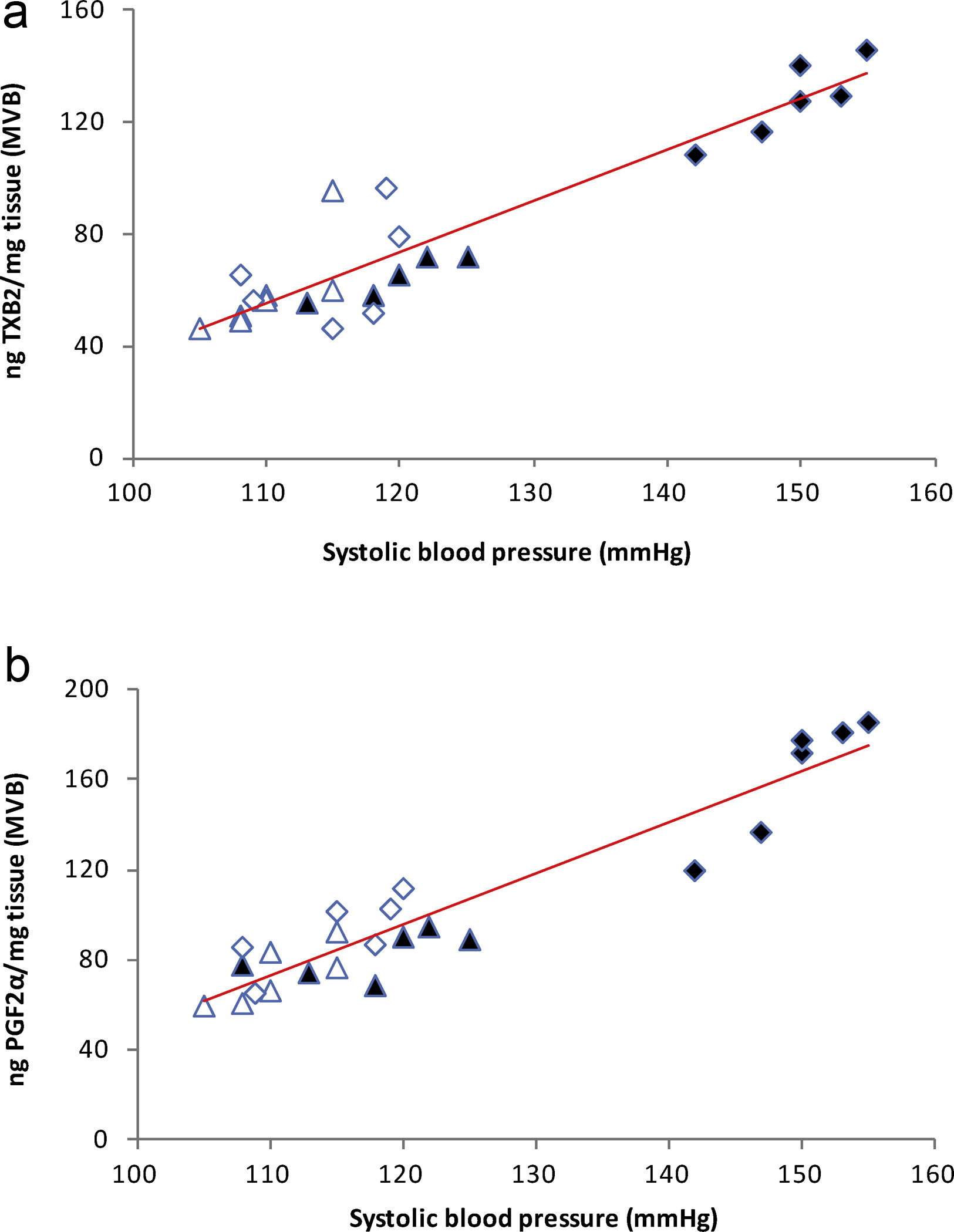
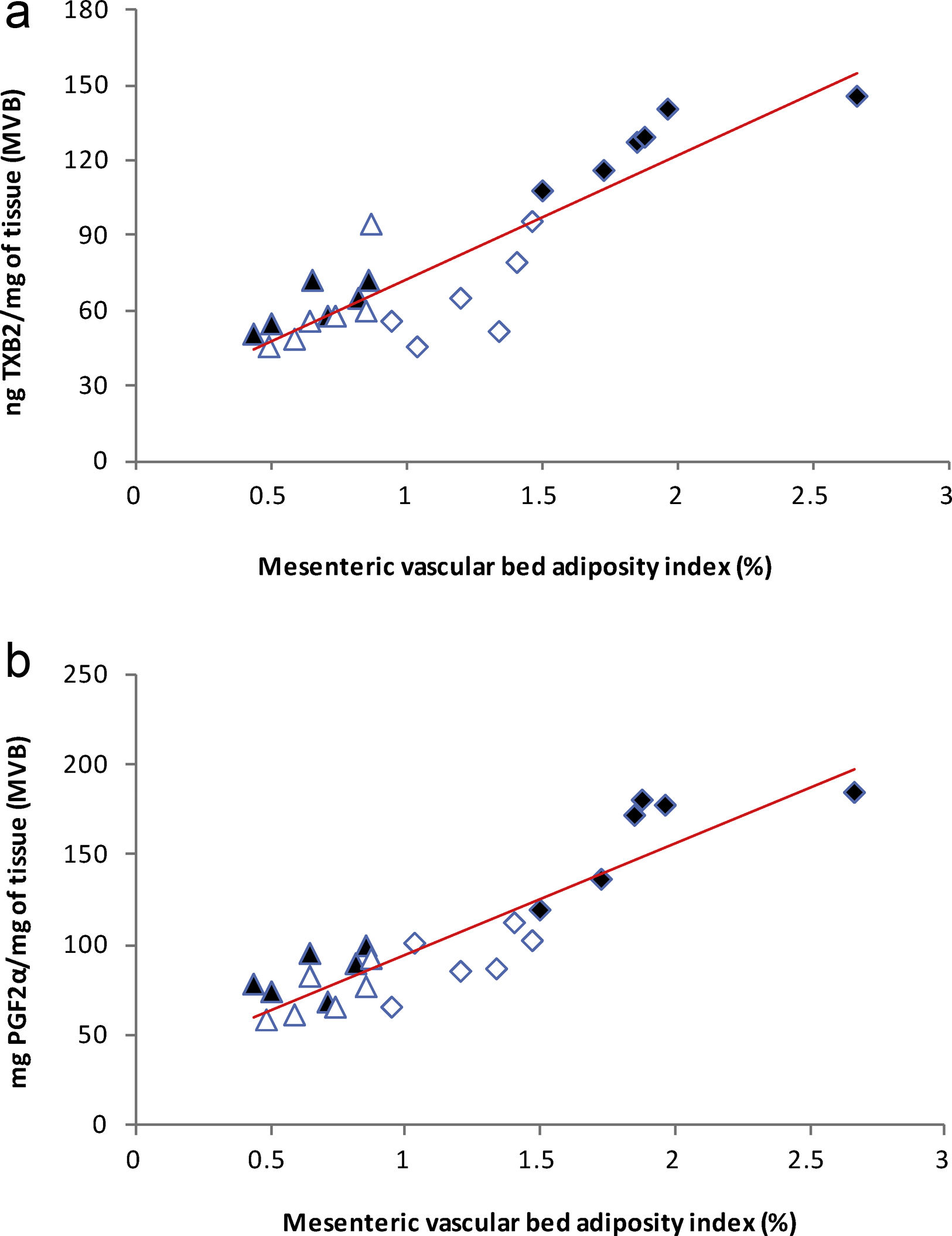
), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.87, R2=0.80, P<0.01. (b) Linear regression of systolic blood pressure against HOMA-IR index: Control (), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.90, R2=0.80, P<0.01. (c) Linear regression of mesenteric vascular bed adiposity index against HOMA-IR index: Control (), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.93, R2=0.86, P<0.01.High-fat diet significantly increased vasoconstrictor prostanoids in HF fed rats compared to C group: TXB2 (P<0.01; Fig. 2a) and PGF2α (P<0.01; Fig. 2b). Losartan prevented these increases in HFL compared to HF rats: TXB2 (P<0.01; Fig. 2a) and PGF2α (P<0.01; Fig. 2b). In addition, positive correlations were found between release of both prostanoids and SBP (TXB2; r=0.93, R2=0.87, P<0.01; Fig. 3a and PGF2α; r=0.95, R2=0.89, P<0.01; Fig. 3b) as well as with mesenteric vascular bed adiposity index (TXB2: r=0.89, R2=0.80, P<0.01; Fig. 4a and PGF2α: r=0.90, R2=0.82, P<0.01; Fig. 4b).
(a) Release of TXB2 in control (C), high-fat diet (HF), losartan-control (CL), losartan- high-fat diet (HFL). *P<0.01 vs. C, CL; **P<0.01 vs. HF. (b) Release of PGF2α in control (C), high-fat diet (HF), losartan-control (CL), losartan- high-fat diet (HFL). *P<0.01 vs. C, CL; **P<0.01 vs. HF.
(a) Linear regression of systolic blood pressure against TXB2 release: Control (
), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.93, R2=0.87, P<0.01. (b) Linear regression of systolic blood pressure against PGF2α release: Control (), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.95, R2=0.89, P<0.01.(a) Linear regression of mesenteric vascular bed adiposity index against TXB2 release: Control (
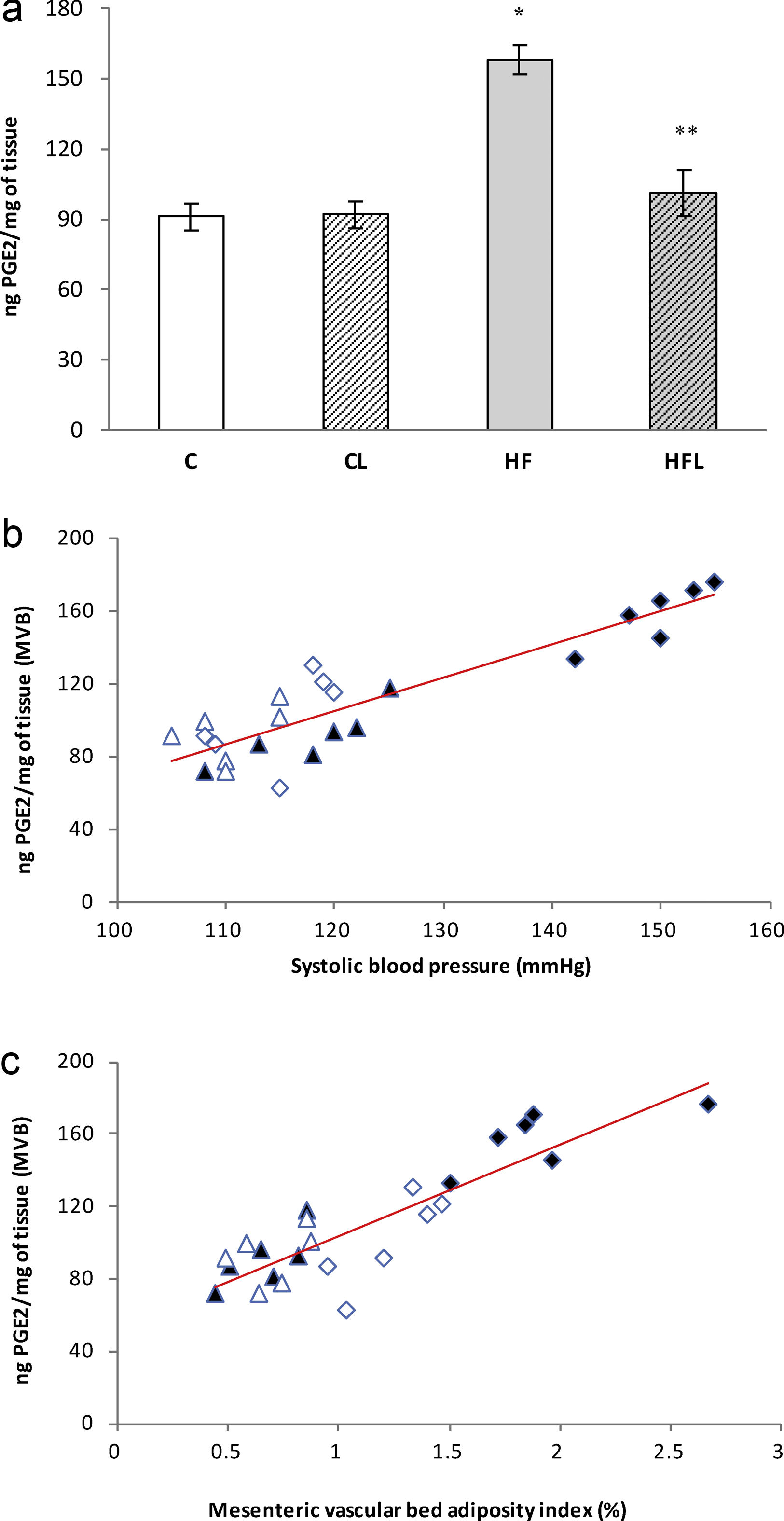
), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.89, R2=0.80, P<0.01. (b) Linear regression of mesenteric vascular bed adiposity index against PGF2α release: Control (), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.90, R2=0.82, P<0.01.As it is shown in Fig. 5a, PGE2 release increased in HF group compared to C (P<0.01) and losartan prevented this effect (P<0.01, Fig. 5a). A positive correlation was found between PGE2 and SBP (r=0.90, R2=0.81, P<0.01; Fig. 5b) and also between PGE2 and adiposity index of mesenteric vascular bed (r=0.88, R2=0.80, P<0.01; Fig. 5c).
(a) Release of PGE2 in control (C), high-fat diet (HF), losartan-control (CL), losartan-high-fat diet (HFL). *P<0.01 vs. C, CL; **P<0.01 vs. HF. (b) Linear regression of systolic blood pressure against PGE2 release: Control (
), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.90, R2=0.81, P<0.01. (c) Linear regression of mesenteric vascular bed adiposity index against PGE2 release: Control (), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.88, R2=0.80, P<0.01.On the other hand, the prostacyclin (PGI2)/thromboxane (TXA2) release ratio (measured as their stable metabolites) was significantly reduced in HF fed rats compared to C group (P<0.01; Fig. 6a). Losartan treatment was able to prevent this reduction in HFL compared to HF (P<0.01; Fig. 6a). Moreover, negative correlations were found between PGI2/TXA2 release ratio and SBP (r=−0.91, R2=0.82, P<0.01; Fig. 6b) as well as with mesenteric vascular bed adiposity index (r=−0.84, R2=0.72, P<0.01; Fig. 6c).
(a) PGI2/TXA2 release ratio in control (C), high-fat diet (HF), losartan-control (CL), losartan-high-fat diet (HFL). *P<0.01 vs. C, CL; **P<0.01 vs. HF. (b) Linear regression of systolic blood pressure against PGI2/TXA2 release ratio: Control (
), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.91, R2=0.82, P<0.01. (c) Linear regression of mesenteric vascular bed adiposity index against PGI2/TXA2 release ratio: Control (), high-fat diet (), losartan-control (), losartan-high-fat diet (). r=0.84, R2=0.72, P<0.01.Our results demonstrate the preventive effect of losartan treatment on the mesenteric vascular bed adiposity deposition as well as on vasoconstrictor (TXB2 and PGF2α) and inflammatory (PGE2) prostanoids release, in a context of hypertension and insulin resistance produced by a high-fat diet. Furthermore, we showed that losartan improved the PGI2/TXA2 ratio, a marker of endothelial dysfunction.
High-fat diet fed rats is a suitable animal model that resembles pathophysiological features of human metabolic syndrome.25 In agreement with previous reports,17,18 we found higher triglyceridaemia, glycaemia, insulinaemia and insulin resistance; increased visceral adiposity and elevated blood pressure. Losartan treatment attenuated such characteristics in HF rats. Previously, Mourant et al.26 reported losartan effects on higher levels of plasma glucose, triglycerides and insulin; except for the fact that losartan treatment was started after 12 weeks of treatment with high-fat diet. In our protocols, dietary and losartan were co-administered from the beginning of the experiments to establish their prevention by pharmacological inhibition of RAS with losartan. On the other hand, they did not measure blood pressure in their study.
Regarding the results on adiposity index, we have found different selection criteria from different authors in defining the fat areas. Some studies reported the sum of epididymal (male rat model), retroperitoneal, perirenal or/and mesenteric fat pads.27-29 Mourand et al.26 had reported losartan effect on the ratio of visceral fat/gastrocnemius muscle considered as an index of body composition but they did not specify where they took visceral fat pads. Wang et al.30 found that losartan treatment exhibited a significantly decreased in epididymal, retroperitoneal and mesenteric fat pad weights in SHR rat model. Our report shows the preventive effect of losartan on the mesenteric vascular bed adiposity increase.
PVAT has been proposed to impact microvascular function and it could be involved in the pathogenesis and progression of insulin resistance and hypertension.31,32 Accordingly, we have found significant correlations between adiposity index of mesenteric vascular bed with insulin resistance and SBP as well. The development of high blood pressure associated with metabolic diseases is multifactorial. One of the possible mechanisms involved could be due to PVAT dysfunction caused by a high-fat diet which produces modifications in both the amount and expression of vasoactive substances,33 thus contributing to susceptibility of the vessels to develop vascular diseases. Within vasoactive substances (vasodilator and vasoconstrictor) implicated in the regulation of vascular tone and resistance can be included, among others, nitric oxide (NO),34 angiotensin II,14 PGs and TXs.35–37
An ectopic fat deposition by high-fat diet may contribute to development of hypertension through increased activity of local RAAS in visceral adipose tissue linked to insulin resistance.38 RAAS activation in PVAT could promote increased angiotensin II, exacerbating insulin resistance.39 Microvascular insulin resistance may be a shared pathophysiological mechanism between hemodynamic and metabolic consequences of visceral adiposity dysfunction.40
There is evidence that supports the role of insulin resistance in the endothelial dysfunction. The local vascular effect of insulin beyond systemic effects, is modulated by production of the vasodilator NO via activation of insulin receptor substrate-1 (IRS-1)/phosphatidylinositol 3-kinase (PI3 kinase) AKT/endothelial NO synthase (eNOS) pathway. Also, increased angiotensin II levels due to PVAT dysfunction can favor microvascular vasoconstriction through AT1 receptor by stimulating the secretion of vasoconstrictors prostanoids.41 The activation of local RAAS in PVAT produce alterations in this signaling resulting in decreased beneficial vascular effects of metabolic insulin.42–44
An upregulated expression of cyclooxygenase (COX) in visceral fat that drive production of vasoconstrictor prostanoids and reactive oxygen species is an important hallmark in the pathogenesis of endothelial dysfunction observed in obesity-related conditions such as metabolic syndrome, hypertension and atherosclerosis.45 Angiotensin II regulates COX-2 expression and prostanoid release via AT1 receptors activation.46,47 It has been reported that losartan reduces COX-2 mRNA up regulation and also acts as a competitive antagonist of the thromboxane A2 receptor.48
In accordance with previous studies,18,19 we found increases of PGE2, PGF2α and TXB2 induced by a high-fat diet at 8 weeks. An increase of pro-inflammatory and contractile substances in PVAT could be associated with the development of insulin resistance, as well as endothelial dysfunction. We have found that adiposity index correlates positively not only with the release of PGE2, but also with vasoconstrictors prostanoids release (PGF2α, TXB2), suggesting a pathophysiological connection. Present results demonstrated a preventive action of losartan on the release of those prostanoids induced by high-fat diet.
It has been reported that losartan reduced TXA2, PGE2 and PGF2α release from second, third and fourth branches of mesenteric artery cleaned of fat in streptozotocin rat model.48 Ishida et al.49 found losartan treatment reduced abnormal release of PGE2 and PGF2α in stimulated rings of the superior mesenteric artery without PVAT. Moreover, Matsumoto et al.50 described that losartan suppressed endothelium stimulated release of prostanoids in mesenteric arteries rings. Wanderer et al.51 found that losartan reduces a PGF2-induced vasoconstriction in basilar artery ring segments after subarachnoid hemorrhage. As far as we know, our results provide new data regarding the preventive effect of losartan on prostanoids release from the entire mesenteric vascular bed in a metabolic syndrome model induce by high-fat diet.
A role for vasoconstrictor prostanoids in hypertension associated metabolic syndrome may be related to increased production of oxygen-derived free radicals determining abnormalities of vascular function that affects expression of eNOS and bioavailability of NO. Moreover, oxidative stress linked to the activation of protein kinase C and the NADPH oxidase regulates prostanoids activity in endothelial dysfunction.52-54 Bayorh et al.55 reported losartan attenuation of oxidative stress induced by glutathione depletion in Sprague-Dawley rats.
Endothelial dysfunction is one of the trigger factors in the pathogenesis of hypertension associated to metabolic syndrome and cardiovascular diseases. We have also demonstrated a decrease in the PGI2/TXA2 ratio, suggesting an endothelial dysfunction in mesenteric vascular bed due to a pro-inflammatory and contractile state of the PVAT exposed to a high-fat diet. Losartan treatment prevented the PGI2/TXA2 ratio alteration induced by high-fat diet.
Finally, our findings attribute, at least in part, endothelial dysfunction in mesenteric vascular bed to alterations on prostanoids release in the context of the triad: adiposity, HOMA IR and hypertension.
ConclusionPVAT dysfunction produce alterations in the release of both vasoconstrictor and vasodilator factors that play a fundamental role in the endothelial dysfunction in hypertension associated to metabolic syndrome. This work has focused mainly on the prostanoids of the mesenteric vascular bed to point out their relevance in vascular function. One of the new potential therapeutic targets to be considered is PVAT. Our experimental findings provide more evidence to choose losartan in the treatment of hypertension in patients considered to be at high risk for developing metabolic diseases. We demonstrated that losartan treatment prevents vasoconstrictor and inflammatory prostanoids release as well as mesenteric vascular bed adiposity increase induced by a high-fat diet. We have shown that losartan plays multiple positive actions/effects beyond its antihypertensive effect.
Funding sourceThis work was supported by the Secretaría de Ciencia y Técnica, Universidad de Buenos Aires Grant number UBACyT 20020170100621BA (2018-2020).
Conflict of interestAll authors have declared no conflict of interest.