Atherosclerosis, a known and prevalent disease, causes progressive deterioration of affected vessels, inducing a blood flow reduction with different complications, and its symptoms usually manifest in advanced stages of the disease. Therefore, the traditional therapeutic alternatives are insufficient because the damage they cause is often irreversible. For this reason, there is a need to implement intelligent forms of drug administration and develop new therapeutic targets that reduce the progression of the atherosclerotic lesion. The implementation of new tools for the prevention, diagnosis and treatment of this cardiovascular disease is of special interest, focusing on achieving a more effective control of the immune system. This review highlights the latest knowledge about nanotechnology as a powerful, modern and promising therapeutic alternative applied to atherosclerotic disease, as well as warning of the potential complications associated with its use.
La aterosclerosis, una conocida enfermedad arterial prevalente, ocasiona el deterioro progresivo de los vasos afectados provocando reducción del flujo sanguíneo con diversas complicaciones, y los síntomas suelen manifestarse en estadios avanzados de la enfermedad. En este sentido, las clásicas alternativas terapéuticas resultan insuficientes debido al carácter muchas veces irreversible del daño provocado. Por lo tanto, emerge la necesidad de implementar novedosas formas más eficaces para administrar fármacos y también el desarrollo de nuevas dianas terapéuticas que reduzcan la progresión de la lesión aterosclerótica. Además, resulta de especial interés la implementación de nuevas herramientas para la prevención, diagnóstico y tratamiento de esta patología cardiovascular, focalizando la atención en lograr un mejor control sobre el sistema inmunológico. En esta revisión se pone en relieve el conocimiento actual sobre la nanotecnología como una alternativa terapéutica potencial, moderna y prometedora, aplicada a la patología aterosclerótica, pero se advierte también sobre posibles complicaciones de su uso.
The prevalence of atherosclerosis has steadily increased worldwide due to population ageing. Economic development and urbanisation have led to habits of diets rich in saturated fats and scant physical exercise, both of which promote and aggravate atherosclerosis.1 However, the development of atherosclerosis is not limited to patients with a western lifestyle characterised by the aforementioned factors. Recent studies have identified novel non-traditional risk factors for atherosclerosis that are all associated with activation of the immune system. These findings are consistent with studies showing inflammation as an essential element in the onset, progression and destabilisation of atherosclerotic plaques. This chronic disease is one of the main causes of serious cardiovascular events, such as myocardial infarction and stroke (responsible for 17 million deaths/year).2,3 Recent research has shown how alterations in the inflammatory pathway can be associated with atherosclerosis.4–9
Atherosclerotic disease is characterised by arterial wall thickening and rigidity, deregulation of lipid metabolism, and the formation of the characteristic plaque. In this situation, restricted blood flow and the eventual rupture of the plaque itself can be life-threatening.10 Plaque formation is a complex biological process that includes endothelial dysfunction, macrophage infiltration, inflammatory factor expression, intraplaque neovascularisation and intima-media thickness remodelling, among other phenomena.11
Atherosclerotic plaque is rich in macrophages, which, together with monocytes, participate in the initiation, progression and destabilisation of atherosclerotic plaques by activating various mechanisms.2 M1 and M2 macrophages in particular play a crucial role in progressive atherosclerotic disease.12 In the early stages of atherosclerosis, macrophages contribute to the clearance of reactive oxidised low-density lipoprotein (oxLDL) particles through scavenger receptor A (SR-A) and CD36-mediated uptake. In later stages, the phagocytic activity of macrophages becomes impaired due to intracellular accumulation of oxLDL. Foam cell macrophages undergo apoptosis due to oxidative stress and inflammatory responses, a process which ultimately contributes to the formation of the pro-thrombotic necrotic core that characterises mature atherosclerotic plaques.2 Some studies have shown that vascular smooth muscle cells within atherosclerotic lesions can switch to a macrophage-like phenotype characterised by higher expression of inflammatory markers that may further contribute to disease progression.13 Furthermore, altered molecular patterns accumulated in atherosclerotic plaques are a dangerous source of factors that can activate macrophages by binding to specific receptors such as Toll-like receptors (TLRs), scavenger receptors (SRs) and intracellular nucleotide-binding oligomerisation domain (NOD)-like receptors (NLRs). Macrophages, therefore, play a pivotal role and modulate the expression of multiple cellular mediators that can create a proinflammatory environment in the plaque (Fig. 1). In addition, lesional macrophages contribute to the remodelling of atherosclerotic plaques into a rupture-prone unstable phenotype by the production of proteases. The presence and proliferation of autoimmune B cells has also been observed within the diseased arterial walls of aged mice with atherosclerotic aortic disease.14 Recent studies have shown that hypercholesterolaemia significantly increases the number of circulating proinflammatory cells, thereby accelerating atherosclerosis,2 while there is evidence that young patients with coronary disease undergoing myocardial revascularisation present high levels of antiphospholipid antibodies. Therefore, besides the conventional risk factors for coronary artery disease, autoimmunity may play an important role in atherosclerotic plaque formation and progression.15
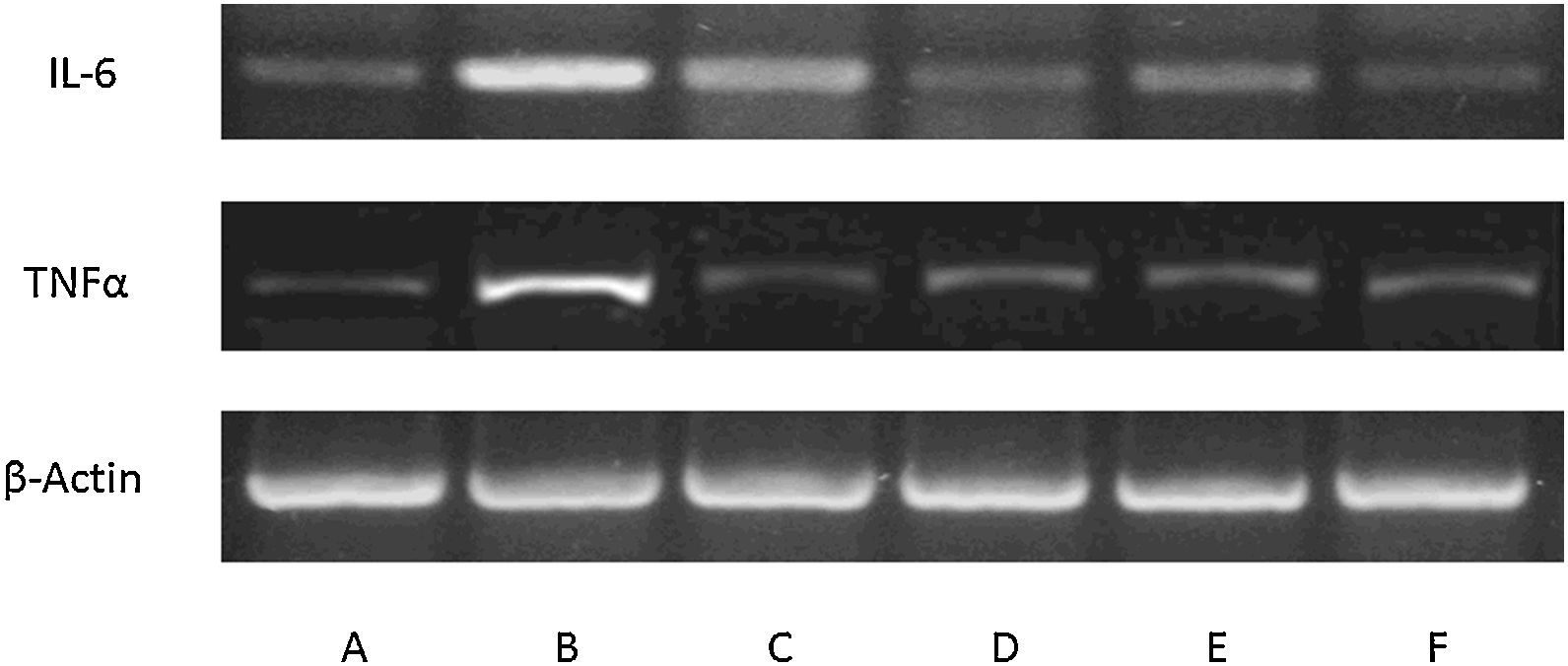
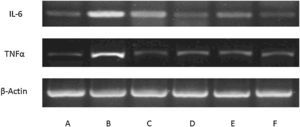
Expression of inflammatory mediators in culture of mouse glial macrophages. Blots showing expression in control macrophages (A), macrophages affected by lipopolysaccharides (BF) and incubated with: allicin (C), phenyl isothiocyanate (D), erucine (E) and indole-3-carbinol (F). The lipopolysaccharide-induced pro-inflammatory response and phytochemical modulation of this response are clearly seen (n=3).
In a parallel development of particular interest for this review, several groups of researchers are designing novel strategies to improve routes of administration for existing drugs, while others are identifying new therapeutic targets to curb the progression of chronic inflammatory diseases, in particular, atherosclerotic lesions. Nanoparticles appear to be an attractive therapeutic tool for delivering anti-inflammatory drugs to specific types of cell in order to attenuate or eliminate immune reactions in certain cell subtypes, such as restricting myeloid cell differentiation and migration of pro-inflammatory monocytes to the plaque.16 Nanotechnology, meanwhile, would increase the bioavailability of poorly soluble compounds, insofar as nanoparticles are dissolved and absorbed by the cell matrix more rapidly than larger molecules. It could also be possible to modify the release kinetics of drugs or contrast agents from the matrix, thus improving dosing regimens or reducing the frequency of administration, and paving the way for a significant improvement in the development of new techniques for diagnosing this disease.17,18
Nanomedicine applied to atherosclerosisNanomedicine is the application of nanotechnology in the treatment, diagnosis, monitoring, and control of biological systems. It is being used for the first time to treat pathologies such as atherosclerosis, and can assist in overcoming delivery barriers of traditional pharmaceutical products. Moreover, the inherent biocompatibility and biodegradability of the compounds used in nanomedicine make this technology an ideal candidate for in vivo administration.10
Studies on macrophage cultures using phosphatidylserine-containing nanostructures in combination with curcumin, an active substance with anti-inflammatory and antioxidant properties, are particularly interesting. These nanostructured lipid carriers significantly inhibited lipid accumulation and pro-inflammatory factor production, and also promoted release of anti-inflammatory cytokines.19
Nanomedicine also has the potential to improve the effectiveness of therapeutic and diagnostic agents, while minimising toxicity and harmful side effects for patients. A recent study clinically evaluated a variety of peptides used to treat, prevent and diagnose atherosclerosis, including peptides designed for cellular targets such as endothelial cells, monocytes and macrophages, smooth muscle cells (SMCs) and platelets, as well as for atheromatous plaque components, such as collagen and fibrin.10
More specifically, a recent study investigated the therapeutic potential of annexin 1-loaded nanoparticles (with anti-inflammatory properties) on atherosclerotic mice. In this experiment, more than 70% of the nanoparticles reached advanced atherosclerotic lesions and showed their potential in treating chronic atherosclerosis by considerably improving plaque properties, including oxidative stress suppression, increasing the production of collagen which promotes the protection of the plaque (associated with a decrease in collagenase activity) and reduced plaque necrosis. Based on these findings, the authors concluded that nanoparticles can be used to carry active substances with specific anti-inflammatory agents and thus stabilise atherosclerotic lesions.20
Furthermore, determination of compositional changes in type i collagen is also an important indicator of plaque progression, and evaluating it makes it possible to correlate the thickness of the fibrous layer and the stability of plaques with atherosclerotic vulnerability. Similarly, the administration of collagen nanoparticles targeting atherosclerotic plaque increased the production of type i collagen, as well as the thickness of the fibrous layer, while decreasing metalloproteinase activity in lesions of the aortic root. As a result, lesion area, necrotic core, and oxidative stress decreased within plaques.10
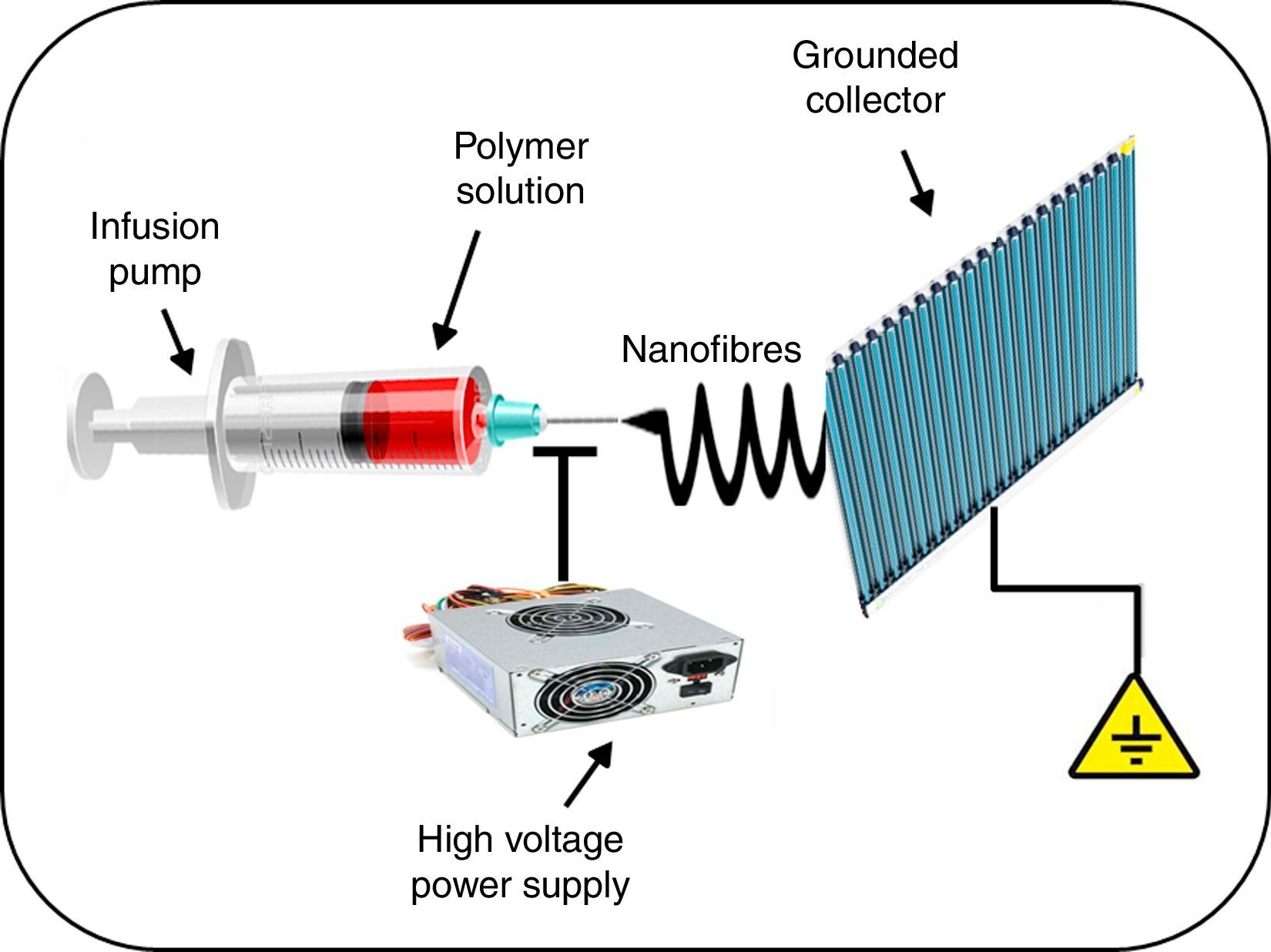
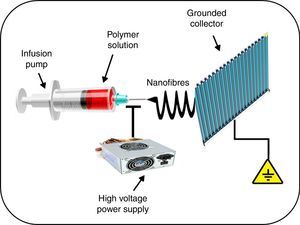
Another interesting application of nanomedicine with regard to the treatment of cardiovascular diseases is related to the solution that nanotechnology offers in the prevention of complications associated with the use of vascular stents. Incomplete endothelialisation, blood cell adhesion to vascular stents, and inflammation of arteries can result in acute stent thrombosis. It has been demonstrated that the systemic administration of acetylsalicylic acid decreases endothelial dysfunction, thus potentially reducing thrombus formation, enhancing vasodilatation, and inhibiting the progression of atherosclerosis.21 Similarly, the administration of non-steroidal anti-inflammatory drugs, such as meclofenamate, mefenamate, flufenamate and aspirin in atherosclerotic mice significantly reduced inflammatory infiltrate in the lesions, while simultaneously decreasing levels of serum steatosis and cholesterol.22 However, these results are overshadowed by the considerable number of side effects in patients receiving this therapy, such as gastrointestinal bleeding. This led a team of investigators to evaluate a hybrid stent with biodegradable nanofibres for the local, sustained delivery of acetylsalicylic acid to injured artery walls. The biodegradable nanofibres are prepared by first dissolving poly(d,l)-lactide-co-glycolide and acetylsalicylic acid in 1,1,1,3,3,3-hexafluoro-2-propanol. The solution is then electrospun into nanofibrous tubes (Fig. 2), which are then mounted onto commercially available bare-metal stents. The experimental results suggest that biodegradable nanofibres release high concentrations of acetylsalicylic acid for approximately 3 weeks. The local delivery of acetylsalicylic acid by these hybrid stents reduced platelet aggregation and monocyte adhesion with minimum inflammation. Therefore, the proposed hybrid stent, with biodegradable acetylsalicylic acid-loaded nanofibres substantially contributed to local, sustained delivery of drugs to promote re-endothelialisation and reduce thrombogenicity in the injured artery.21
Another study made a similar contribution with the development of a biodegradable dual drug-eluting stent with sequential-like and sustainable drug-release of the antiplatelet action of acetylsalicylic acid together with paclitaxel, which inhibits smooth muscle cell proliferation. The aim of the device is to attenuate stent-induced vascular lesions.23
Among the advantages of drug-eluting stents over conventional systemic pharmacotherapy is their ability to deliver higher drug concentrations directly to the site of the lesion, with minimum systemic side effects. Nanofibres, for their part, induce a very low inflammatory reaction in vascular tissues and are completely absorbed in 4 weeks.21
Another potentially significant application of nanotechnology in the treatment of atherosclerosis involves combining nanofibres with stem cell therapy for tissue regeneration.24
In recent years, a new paradigm on the specific orientation of functionalised nanoparticles has been generated. By attaching antibodies, proteins, peptides or other ligands to its surface, a nanoparticle can be targeted to single or multiple receptors that are expressed on the surface of (or inside) an atherosclerotic plaque. For example, vascular targeting can be accomplished using nanoparticles that have been functionalised with specific ligands to adhesion molecules such as VCAM1, selectins or integrins such as αVβ3, as these adhesion molecules are expressed on the activated luminal endothelium of the affected blood vessel. Once the nanoparticle is attached to the specific receptor being targeted, it can either bind to endothelial cells or become internalised. The use of functionalised nanoparticles, moreover, does not increase the percentage of the administered dose of the nanoparticle that reaches the specific lesion, but does improve distribution of the nanoparticle among pathological lesions and increase its uptake by endothelial cells. The physicochemical properties of nanoparticles will largely determine their biodistribution and intracellular uptake, thus increasing their therapeutic activity.25
Nanotechnology for the diagnosis of atherosclerosisIn addition to their proposed novel therapeutic use, nanoparticles can be combined with diagnostic imaging agents (namely, fluorophores, chelated ions and metals, among others). Nanoparticle-carrying contrast agents appear to progressively improve the diagnosis of atherosclerosis, since the formation of these specific molecular images is superior to traditional imaging contrast agents.10
The development of non-invasive techniques for monitoring and diagnosing atherosclerotic plaques would give a more accurate picture of these lesions. These techniques would also be able to provide a more comprehensive and dynamic evaluation of the therapeutic efficacy of different atherosclerosis treatments. An interesting example is magnetic resonance, which emerges as a promising non-invasive method for the formation of imaging atherosclerotic and vessel wall lesions of the artery, with excellent spatial resolution.11
The most extensively studied class of magnetic nanoparticles for contrast agent development are iron oxide nanoparticles, also known as ferrite nanoparticles. This is due to their superparamagnetic nature, biodegradability, inoffensive toxicity profile, and their reactive surface that is readily modifiable with different biocompatible coatings.26
Another area of interest involves profilin-1, a type of small actin-binding protein, which is involved in the modulation of cytoskeleton polymerisation and reorganisation. Recent research shows that profilin-1 over-expression triggers the onset and development of atherosclerotic disease, mainly through regulating the proliferation and migration of vascular smooth muscle cells. Therefore, profilin-1 is recognised as a promising potential molecular target of atherosclerosis, and for this reason was applied to DMSA (dimercaptosuccinic acid)-Fe3O4 nanoparticles to obtain in vivo magnetic resonance images of atherosclerotic plaques.11
Some studies have also shown that macrophages of the M1 inflammatory phenotype can selectively target nanostructures by model hybrid lipid–latex (LiLa) nanoparticles bearing phagocytic signals. As a result, LiLa nanoparticles can target M1 macrophages, thus allowing non-invasive imaging of the atherosclerotic plaque by MRI. Taken together, these results suggest that phagocytic signals can preferentially target inflammatory macrophages in experimental models of atherosclerosis, thus opening up the possibility of future clinical applications that diagnose and/or treat these conditions. However, due to the poor biodegradability of latex, LiLa nanoparticles are unlikely to be used in human patients. Nevertheless, the principles of phagocytic targeting used in the above-mentioned studies can be generalised to any other biocompatible and biodegradable nanoparticles.27
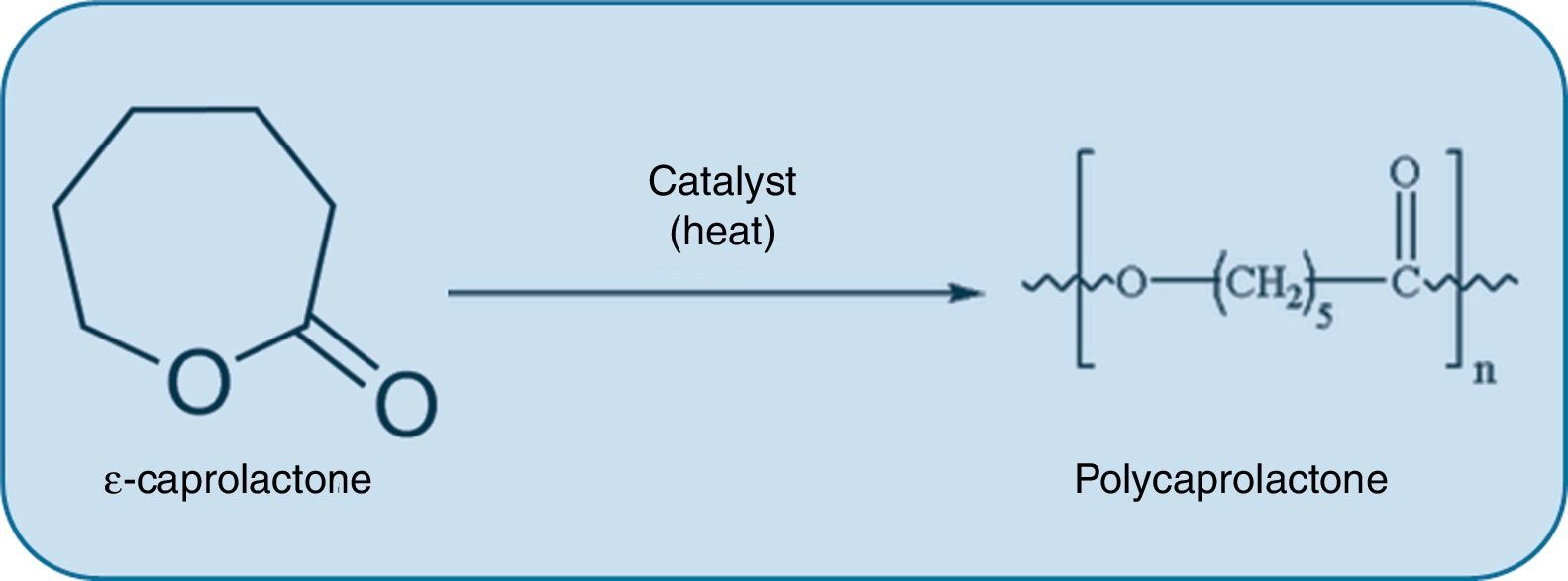
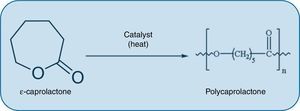
Disadvantages of nanomedicine for the treatment of atherosclerosisIn the same way in which the potential benefits of nanotechnological applications in atherosclerotic disease are considered, some disadvantages should also be contemplated. For example, the use of nanotechnology can be detrimental specifically in the context of certain tissue engineering techniques that permit the design of bioresorbable grafts which are implanted to restore the function of arteries damaged by atherosclerosis. These grafts offer the possibility of creating a synthetic conduit that resists thrombogenesis and ultimately transforms into neotissue that is comparable to the native artery and capable of growth, remodelling and repair. However, these implants are susceptible to calcification during the remodelling process, which implies a potentially fatal complication in the long-term. Interestingly, unlike nanometric-pore polylactic acid (PLA) grafts, large-pore PLA grafts with a polycaprolactone (PCL) coating (Fig. 3) induce a well-organised neointima and prevent calcification.28
Administration of nanoparticles to healthy animals significantly impairs vasodilator responses in coronary arterioles and mesenteric microvascular function. In vitro, iron oxide nanoparticles induced cytoplasmic vacuolation, mitochondrial swelling, and cell death in human aortic endothelial cells, and stimulated nitric oxide production and the adhesion of monocytes. These results indicate that nanoparticle exposure is not only a risk factor for the acquisition of atherosclerotic disease, but may also threaten individuals who already suffer from these conditions.29 Nanotechnology, therefore, involves the creation of new materials with characteristics that are difficult to predict based on current knowledge. Some of the vast array of materials developed are toxic to biological systems.30 Intravenous injection of multi-walled carbon nanotubes (MWCNTs), in rat models fed a diet rich in lipids and vitamin D3, resulted in the development of atherosclerosis aggravated by a greater, calcified aortic lesion. Disrupted endothelial tight junctions following exposure to MWCNTs indicated that increased turbulence and altered endothelial function may trigger atherosclerosis.29 This illustrates the importance of gathering more information on the toxicity and biokinetics of nanoproducts and deepening our understanding of the intentional delivery of these materials for medicinal purposes and the effect of unintentional environmental exposure on humans. This will give a more accurate picture of the real risks of using nanomaterials.31
Many studies have concluded that the aetiology of atherosclerotic disease may be determined by factors such as oxidative stress, inflammation, and damage to mitochondrial DNA and vascular endothelial cells. These studies have also suggested that nanoparticle exposure could aggravate all of these potential triggering factors, and consequently accelerate atherosclerosis progression through platelet aggregation and vascular thrombosis. Therefore, although nanoparticles hold promise as targeted drug delivery carriers to treat atherosclerosis, particular attention should be paid to their potential adverse effects.29
However, most polymers used in medicine are generally biocompatible. Furthermore, when developing safe biomedical nanotechnologies, it is essential to carefully evaluate the interactions between nanoparticles and the components of the immune system. This is because many polymeric materials used in numerous biomedical nanostructures are, logically, capable of triggering various immune reactions in the body, because molecular patterns on the surface of these exogenous materials are foreign to the immune system. Thus, we can start to gain a better understanding of the basic principles which enable the rational design of safe and useful nanomaterials.32
Finally, ectopic calcification is associated with several human diseases, including atherosclerosis,33–35 and mineral nanoparticles have been detected in soft tissue with signs of ectopic calcification.36 In a recent study, Bertazzo et al.37 demonstrated the presence of mineral nanoparticles in aortic valves and atherosclerotic coronary arteries. These particles form in biological fluids when calcium and phosphate concentrations exceed saturation.
Conclusions and perspectivesIt is essential to broaden our current understanding of the prevention, diagnosis and treatment of atherosclerosis, since this disease can be triggered not only by an unhealthy lifestyle and lack of physical exercise, but also by other non-traditional factors fundamentally related to the immune system.
Nanotechnology and pharmacology are two scientific fields that can be used both individually and jointly to design new, more comprehensive therapeutic alternatives to analyse deadly, multifactorial diseases, such as atherosclerosis. Examples of the contributions to conventional treatments include the use of nanoparticles to carry anti-inflammatory drugs, improvement in the efficacy of stents, stem cell combination, targeted vectorisation of specific molecules, while the combination of nanostructures with fluorophores or other substances to improve diagnosis of atherosclerosis is a promising and highly applicable field of study. Finally, the disadvantages and potentially harmful effects of these new methodologies must also be borne in mind.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were conducted on human beings or animals for this research.
Data confidentialityThe authors declare that they have followed the protocols implemented in their place of work regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingWe thank the following organisations for their contributions to the research, authorship and publication of this article: Consejo de Investigación y Tecnología de la Universidad de Cuyo (SECyT) [Research and Technology Council of the Universidad de Cuyo], Mendoza, Argentina, and Agencia Nacional de la Investigación Científica y Técnica (ANPCyT) [National Agency of Scientific and Technical Research]; both grants were awarded to Walter Manucha. Grant PICT 2012-0234, BID 2777 OC/AR.
Conflicts of interestNone of the authors has any conflict of interest to declare regarding the content of the paper.
Please cite this article as: Martín Giménez VM, Ruiz-Roso MB, Camargo AB, Kassuha D, Manucha W. Nanotecnología, un nuevo paradigma en el tratamiento de la aterosclerosis. Clin Investig Arterioscler. 2017;29:224–230