Over time, interest in fibrates has gone hand in hand with scientific evidence of the role of triglyceride-rich lipoproteins in the development of atherosclerosis and related cardiovascular disease. Although there is no doubt that statin therapy has brought about a reduction in cardiovascular risk, it is at most 30%–35%, leaving a remaining cardiovascular risk of 70% in statin-treated patients, not directly related to LDL-cholesterol (LDL-C), without adequate control. If we exclude the particular case of lipoprotein(a) [Lp(a)], this segment would include two other lipid risk factors, hypertriglyceridaemia and low HDL cholesterol (HDL-C) concentrations, often associated with metabolic syndrome or type 2 diabetes mellitus (T2DM). To date, from the point of view of cardiovascular risk reduction, pharmacological measures aimed at increasing HDL-C have been ineffective, as demonstrated by the failure of cholesteryl ester transfer protein (CETP) inhibitors. Consequently, control of hypertriglyceridaemia remains a possible intervention to reduce cardiovascular risk in statin-treated patients,1 hence the renewed interest in fibrates.
Why are plasma triglycerides a cardiovascular risk factor?Plasma triglycerides are transported in lipoproteins that contain, as a structural apolipoprotein, apolipoprotein B, apo B-100 when the origin is hepatic (VLDL), in fasting situations, or apo B-48, when the origin is intestinal (chylomicrons), in postprandial situations. Both chylomicrons and nascent VLDL are too large to be incorporated into the subendothelial space of the arterial system. However, both types of lipoproteins are extensively remodelled during their stay in the blood compartment by lipolytic processes that deliver fatty acids to various tissues, such as skeletal muscle and adipose. During these processes, they are transformed into intermediate or remnant lipoproteins, changing their apolipoprotein content and lipid composition, proportionally reducing their triglyceride content, and enriching their cholesterol content, finally giving rise, in the case of VLDL, to LDL lipoproteins, which contain only apo B-100. These changes mean that the remaining lipoproteins have high atherogenic potential, due to a series of characteristics, such as a smaller size and a higher cholesterol content, and because the fatty acids present in the remaining lipoprotein triglycerides, and their derivatives by enzymatic modification, redox processes, etc., have the capacity to promote chronic low-intensity inflammatory processes that favour the development of atheromatous plaque.2–4
Fibrates and hypertriglyceridaemiaTogether with ion exchange resins, fibrates constitute one of the first therapeutic groups used in the management of dyslipidaemia. Although they favourably modify concentrations of the three major lipoproteins (VLDL, LDL, and HDL), their most marked effect lies in the reduction of plasma triglycerides, which is why they have traditionally been used in the treatment of hypertriglyceridaemia and mixed or atherogenic dyslipidaemia, typical of diabetics. The three most commonly used fibrates are gemfibrozil, bezafibrate, and fenofibrate. In the pre-statin era, gemfibrozil demonstrated a reduction in the risk of cardiovascular events, both in primary and secondary prevention, but its use has declined because its administration in combination with statins alters the metabolism of statins (especially simvastatin), increasing the occurrence of adverse reactions associated with skeletal muscle toxicity. Bezafibrate and fenofibrate have not been widely used in the statin era to manage dyslipidaemia for two basic reasons:
- □
Lack of efficacy in reducing cardiovascular accidents when used in combination with statins.
- □
The impaired safety profile of the statin-fibrate combination, due to potential hepatic and renal adverse effects associated with the use of fibrates.
Fibrates act as selective ligands of a type II nuclear receptor (transcription factors activated by binding to specific ligands), the peroxisome proliferator-activated receptor alpha or PPARα. Among the traditional fibrates, gemfibrozil and fenofibrate primarily have an affinity for PPARα, while bezafibrate has a similar affinity for all three PPAR isoforms, α, β, and γ. Binding of the ligand to the PPARα receptor promotes its activity, increasing:
- □
The transactivation activity of genes with a PPAR-responsive element in their promoter area, such as APOAI, APOAV, LPL, ACO, or CPTI, increasing lipolytic activity on plasma lipoproteins and fatty acid catabolism in the liver.
- □
Transrepression activity on pro-inflammatory transcription factors, such as NFκB or AP-1, thus reducing the low-intensity inflammatory processes associated with the chronic alterations in energy metabolism present in metabolic syndrome.5
Although, as already indicated, fibrates have not shown a reduction in cardiovascular events in association with statins, the specific study of subgroups of patients included in the FIELD and ACCORD trials, in which fenofibrate was used, indicate a possible significant reduction in patients with high triglyceride and low HDL-C levels, a situation known as atherogenic dyslipaemia, characteristic of patients with T2DM or metabolic syndrome.6,7
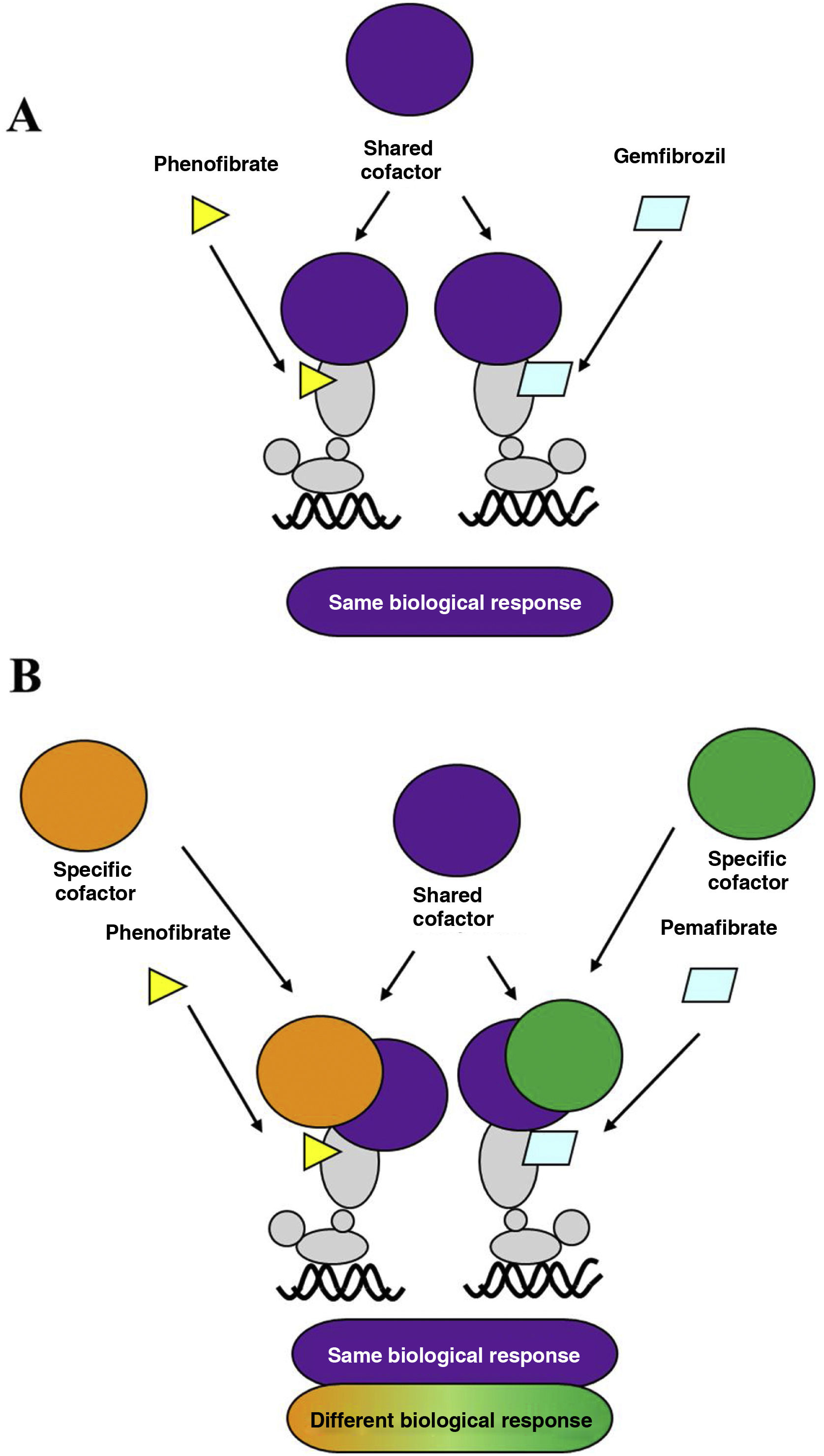
Over the last decade, a new fibrate, pemafibrate, has been developed that has a high affinity for the PPARα receptor, a strong hypotriglyceridemic effect, and a good safety profile, which has led to its approval by the Japanese health authorities for the control of dyslipidaemia in combination with statins. Pemafibrate, which is excreted via the biliary route, unlike fenofibrate, which is mostly excreted in the urine, has been classified as a selective modulator of the PPARα receptor, compared to classical ligands such as fenofibrate.8 Due to its specific molecular interaction with the ligand binding site or LBD of PPARα, it favours its association with a transcriptional coactivator protein complex that enhances the expression of the genes responsible for the lipid-lowering effect, while reducing the intensity of the adverse hepatic and renal effects typical of fibrates. Unlike fenofibrate, pemafibrate induces in human hepatocytes, in a PPARα-dependent manner, the expression of genes such as ACO, VLDLR, FGF21, or ABCA1, positively modulating lipid metabolism (Fig. 1).
A. Classical PPARα receptor ligand: both ligands (fenofibrate and gemfibrozil) recruit the same transcription co-activators and produce similar effects. B. PPARα receptor modulator: pemafibrate shares coactivators with the classical ligand (fenofibrate), but also recruits different coactivators, thus presenting a different efficacy and safety profile to fenofibrate.
These particular characteristics of pemafibrate have led to its clinical use being associated with a clear improvement in the efficacy/risk ratio compared to traditional fibrates, especially fenofibrate, leading to the development of the PROMINENT clinical trial,9 the intention of which was to verify the possible effect of pemafibrate in reducing cardiovascular risk in diabetic patients. PROMINENT is a randomised, double-blind, multinational clinical trial involving more than 10,000 patients with T2DM who had hypertriglyceridaemia (200–499 mg/dL) and HDL-C ≤ 40 mg/dL, mostly treated with statins and with LDL-C ≤ 80 mg/dL. The primary endpoint of the trial was the aggregate incidence of myocardial infarction, ischaemic stroke, coronary revascularisation, and cardiovascular death. During the 3.4 years of mean follow-up, pemafibrate treatment did not reduce the primary endpoint compared to the placebo group, despite inducing a marked reduction in triglycerides, cholesterol contained in VLDL and remnant lipoproteins, and circulating apo C-III. In contrast to the clinical trials conducted in the authorisation process for pemafibrate (in which it was not associated with statins), the PROMINENT study detected a modest but significant increase in apo B (4.8%) and LDL-C (12.3%) concentrations in patients treated with pemafibrate compared to the placebo group, suggesting that the lipolytic capacity to convert VLDL to LDL, increased by pemafibrate, would exceed the hepatic LDL clearance capacity in these patients. In this regard, the results of a phase III trial comparing the effect of pemafibrate at two doses, .2 and .4 mg/day, compared to fenofibrate (107 mg/day) are interesting. Although pemafibrate, at both doses, showed a superior hypotriglyceridemic effect to fenofibrate, at the .4 mg/day dose (the dose used in the PROMINENT study), it increased LDL-C levels by 4% (p = .054) over the fenofibrate group.10 The results of the PROMINENT trial, published in November 2022,9 dealt a severe blow to the theory of vascular risk associated with triglyceride-rich lipoproteins and the possible use of pemafibrate to reduce it in patients already treated with statins. Given that LDL-C concentration is the main cardiovascular risk factor, the increase in LDL-C values observed in the PROMINENT trial could have been sufficient to negate the potential beneficial effects of the reduction in plasma triglyceride concentration, remnant lipoproteins or anti-inflammatory activity associated with pemafibrate treatment. In this regard, it should be noted that in the ACCORD study, in which, as in the PROMINENT study, the patients included were mostly treated with statins, the additional use of fenofibrate (initial dose of 160 mg/day) did not lead to any significant change in LDL-C concentration compared to the placebo group (reductions from 100.0 to 81.1 mg/dL in the fenofibrate group and from 101.1 to 80.0 mg/dL in the placebo group at the end of the study).
Pemafibrate: forget or reposition?A cursory analysis of the results of the PROMINENT study would indicate that pemafibrate might be destined for the list of new therapies in cardiovascular prevention that did not live up to expectations, headed by the CETP inhibitors. However, closer scrutiny indicates that the use of pemafibrate, despite the slight increase in LDL-C levels, does not significantly increase cardiovascular risk, nor does it worsen the overall incidence of adverse effects compared to placebo. Although the results of the PROMINENT study suggest no difference in renal toxicity and venous thrombosis incidence compared to fenofibrate, pemafibrate does significantly improve hepatic safety, significantly reducing the total number of hepatic adverse events. Pemafibrate did not increase transaminase levels compared to placebo and, in fact, significantly reduced the incidence of non-alcoholic fatty liver disease (NAFLD) by 22%. The latter effect is of paramount importance for two reasons:
According to a recent study, NAFLD has a prevalence of 32.4% worldwide and is on the rise.11 It should be emphasised that, to date, NAFLD has no approved pharmacological treatment and is the gateway to the future development of cirrhosis and hepatocellular carcinoma, diseases that both have a difficult prognosis.
NAFLD is now considered a cardiovascular risk factor, because it is associated with increased cardiovascular morbidity and mortality.12,13
Recently, Nakajima et al. reported the results of a phase II trial in patients with steatohepatitis (118 patients), in which pemafibrate treatment reduced liver stiffness, as assessed by magnetic resonance elastography, and blood levels of LDL-C and ALT vs. placebo.14 The repositioning of pemafibrate to treat NAFLD could not only be an effective therapy for NAFLD, but could also help reduce cardiovascular risk in NAFLD patients.
Although approximately 50% of patients with obesity and/or T2DM are considered to have NAFLD, this does not seem to be the case for the patients participating in the PROMINENT study (200 cases in more than 5000 patients included in the placebo group, prevalence less than 5%), because patients with liver disease with clinical symptoms were in fact excluded from the study. It remains to be seen what the outcome of the PROMINENT study would have been if NAFLD had been included as an inclusion criterion.
FundingFunding was obtained from grant PID2020-112870RB-I00 from MCIN/AEI/10.13039/501100011033, grant 2021 SGR 00345 from the Generalitat de Catalunya, there is a basic research grant 2020 from the SEA. R.B is a pre-doctoral student PREDOCS-UB.
Conflict of interestsThe authors have no conflict of interests to declare.