Atherosclerosis, one of the main pathologic entities considered epidemic and a worldwide public health problem, is currently under constant review as regards its basic determining mechanisms and therapeutic possibilities. In this regard, all patients afflicted with the disease exhibit mitochondrial dysfunction, oxidative stress and inflammation. Interestingly, nitric oxide – a known vasoactive messenger gas – has been closely related to the inflammatory, oxidative and mitochondrial dysfunctional process that characterises atherosclerosis. In addition, it has recently been demonstrated that alterations in the bioavailability of nitric oxide would induce the expression of heat shock proteins. This agrees with the use of functional foods as a strategy to prevent both vascular ageing and the development of atherosclerosis. Finally, a greater knowledge regarding the mechanisms implied in the development of atherosclerosis will enable proposing new and possible hygiene, health and therapeutic interventions.
En la actualidad se encuentran en constante revisión los mecanismos determinantes primarios y las posibles terapéuticas de una de las principales entidades patológicas considerada epidémica y constituida como problema de salud pública mundial: la aterosclerosis. En tal sentido, pacientes que la padecen presentan como común denominador disfunción mitocondrial, estrés oxidativo e inflamación. De especial interés, el óxido nítrico, un conocido gas mensajero vasoactivo, ha sido estrechamente relacionado con el proceso inflamatorio, oxidativo y disfuncional mitocondrial propio de la aterosclerosis. Por otro lado, muy recientemente se ha demostrado que alteraciones en la biodisponibilidad del óxido nítrico inducirían la expresión de proteínas de shock térmico. Este mecanismo sería inducido también por el uso de los denominados alimentos funcionales como estrategia para prevenir el envejecimiento vascular así como el desarrollo de aterosclerosis. Finalmente, el mayor conocimiento de los mecanismos implicados en el desarrollo de la aterosclerosis nos permitirá proponer nuevas y posibles intervenciones higiénicas, sanitarias y terapéuticas.
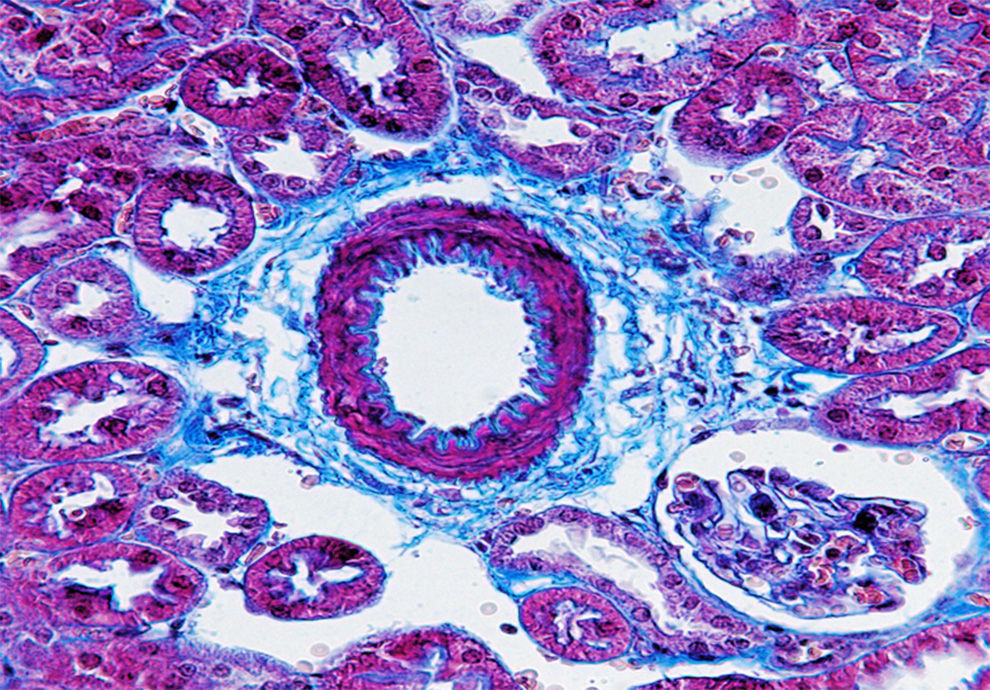
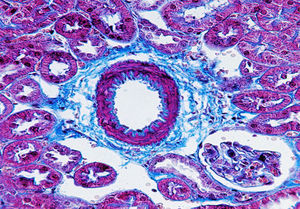
Atherosclerosis as an emerging pathological condition in industrialised countries corresponds to a syndrome characterised by the deposit and infiltration of lipid substances on the walls of medium- and large-diameter arteries. Furthermore, it causes smooth muscle cell inflammation, multiplication, and migration leading to a thinning of the artery lumen,1 ultimately leading to the onset of cardiovascular diseases (CVD). During the chronic inflammatory process of atherosclerosis itself, the tissue damage and repair can occur simultaneously and connective tissue deposits cause fibrosis with an abnormal amount of connective tissue (Fig. 1).
Collagen fibres stained using the Masson technique. Representative image: vessel coming from a kidney slice from a mouse deficient in apolipoprotein E as a model of atherosclerosis. The extracellular fibre matrix deposit in the connective tissue is seen in blue. Fibrosis is observed with an increased extracellular matrix deposit. Magnification 400×.
Interestingly, the World Health Organisation has called CVDs a neglected worldwide epidemic and stressed that they are present in all countries, with the exception of some very poor ones. It also warned that they represent a serious threat to public health, with a large impact on social and economic development. The risk factors that aggravate it serve as population health indicators, and five of them are closely linked to non-communicable diseases, these being hypertension, tobacco use, alcohol consumption, hypercholesterolaemia, and obesity or overweightness.2 These risk factors encourage the development of the inflammatory process of atherosclerosis.3–6
The worldwide impact on public health and the atherosclerosis projections justify a permanent ramping-up of studies aiming to better understand the primary determining mechanisms as well as their therapies. In this sense, patients with atherosclerosis present mitochondrial dysfunction and oxidative stress and inflammation as a common denominator.7,8 Oxidative stress in combination with a lower bioavailability of nitric oxide (NO) seems to play a key role in the pathogenesis of atherosclerosis, vascular inflammation, and endothelial dysfunction.9 In keeping with and of special interest for this review, NO, a known vasoactive messenger gas, has recently been related to the inflammatory, oxidative and mitochondrial dysfunction processes of atherosclerosis.10
Furthermore, our working group demonstrated, in line with other laboratories, that an increased bioavailability of NO induces the expression of heat shock proteins (HSP).11–16 HSPs are a highly conserved family of proteins with several functions expressed by all cells exposed to environmental stress. The most recent studies show that certain HSPs may be potential risk markers for atherosclerosis and related CVDs,17 while others, such as heat shock protein 70 (Hsp70), seem to be directly related to protective effects on atherosclerosis development and maintenance.18 Inducing these proteins would also be positively modulated by using so-called functional foods as a strategy to prevent vascular ageing and the development of atherosclerosis.19 This is consistent since it is known that the Western diet is associated with the chronic inflammatory process involved in all stages of atherosclerosis development, and it is also increasingly recognised as a universal mechanism for developing several chronic, degenerative diseases, such as autoimmune diseases, some cancers, and even osteoporosis.20
The concept of functional foods was originally introduced in the East, more than three decades ago, by healthcare authorities to guarantee a better quality of life vis-à-vis increased longevity. Thus a new understanding of foods developed specifically to improve health and reduce the risk of contracting diseases was proposed.21 This includes those foods containing certain minerals, vitamins, fatty acids, or dietary fibre, foods to which biologically active substances have been added such as phytochemicals or other antioxidants, and probiotics, which contain live cultures of beneficial microorganisms.22 As a result, natural products are gaining greater popularity for combating several threats, including oxidative stress, cardiovascular diseases, cancer, and even immune dysfunction, among the most notable. Specifically regarding CVD, the use of functional and/or nutraceutical foods account for new contributions to understanding, preventing, and possibly treating it. In this regard, garlic has a privileged position in history and was recognised for its therapeutic potential.23 Moreover, it was recently demonstrated that allicin (biologically active compound of garlic) has a protective effect associated with reduced oxidative stress, NO modulation, and significantly increased Hsp70.24 This is significant since it is known that endothelial Nitric Oxide Synthase (eNOS) dysfunction is the primary cause of atherosclerosis and that certain HSPs inhibit smooth muscle cell proliferation and would participate in vascular repair.25
The aetiology of the main CVDs such as atherosclerosis is still not fully elucidated. Nevertheless, recent evidence suggests that NO and HSPs such as Hsp70 are key elements in the oxidative stress process underlying these diseases. Furthermore, an emerging role was recently suggested for the NO and Hsp70 pathways linked to the use of functional foods, which is of particular interest for the current state of knowledge. Therefore, a better comprehension of the mechanisms involved in developing atherosclerosis will enable us to propose new possible hygiene, health, and therapeutic interventions.
Nitric oxide and Hsp70 in atherosclerosisNO, a reactive nitrogen species with an ultrashort half-life, is a regulating factor for a diverse range of physiological functions such as controlling vascular resistance, neurotransmission, and modulating inflammatory processes, among others.11,26–30 Biochemically and in the cytosol, NO is a molecule synthesised by NOS enzymes with l-arginine, NADPH, and O2 as basic substrates.31 Furthermore, mitochondrial production has also been described, regulated by a local isoform of NOS (mNOS) and/or by non-enzymatic reactions with O2 and ubiquinol (UQH2).32 Compared to other reactive substances, NO has a high diffusion in biological systems given its lipophilic nature, neutral charge, and relatively low reactivity.33 In the mitochondrial matrix, NO reacts with the superoxide anion (O2−) resulting in peroxynitrite (ONOO), which is a potent cytotoxic that is easily diffused from the intramitochondrial space.34 Both NO and ONOO are pro-oxidants that can lead to oxidative stress and cell damage due to oxidation and nitration of lipids, proteins, DNA, and deteriorated mitochondrial function.35
NO is generated throughout the body by different isoforms of the NOS enzyme, and local production is what determines its physiological actions. Specifically, the endothelial isoform (eNOS) is abundant in the vascular endothelium, but it is also found in cardiomyocytes, neurons, epithelial cells, adipocytes, and hepatocytes.36,37 The inducible isoform (iNOS), which has the largest capacity for generating NO, is – as its name suggests – inducible and is expressed in several types of cells such as macrophages, in response to inflammatory stimuli from cytokines, lipopolysaccharides, and other immunological agents. iNOS expression is regulated at the transcriptional and post-transcriptional level by signalling pathways that involve mediators such as NF-kappa B factor or MAPK.38 Lastly, nNOS is expressed especially in neurons, skeletal muscle, and epithelial cells. It is a Ca2/calmodulin-dependent isoform that can be activated by N-methyl-d-aspartate receptor agonists.39 Unlike the inducible isoform, both the neuronal and endothelial isoforms are expressed constitutively, but their activity is regulated by the intracellular calcium concentration. As a result, the iNOS enzyme, as well as NO itself, are involved in a variety of acute and/or chronic pathological states such as inflammation, ischaemia-reperfusion, diabetes, cancer, neurological disease, ageing, and kidney disease.40–44 Accordingly, and despite the controversies on the toxic or beneficial effects of NO, our group reported, with no precedent, a decrease in the bioavailability of NO with a lower expression of iNOS associated with an Hsp70 deficit and significant induction of apoptosis in a kidney disease model. Meanwhile, administering l-arginine in vivo induced the expression of Hsp70 leading to less apoptosis and reduced NADPH activity. We also verified an increase in Bcl2 and Hsp70 interaction. These findings suggested that NO can cause resistance to the cell death provoked by the kidney disease model by modulating mitochondrial signals through Hsp70 induction.45
Hsp70 is one of the most ubiquitous and highly conserved HSPs. HSPs are molecular chaperones comprised of polypeptide families that help damaged molecules recover their functional conformation. They are synthesised in response to different stress factors, such as heat, hypothermia, hypoxia, free radicals, ischaemia, ethanol, ultraviolet radiation, and viral infection, among the most studied.46 They also protect proteins, lipids, and nucleic acids from damage by reducing oxidation and they can modulate cell function and gene expression by contributing to protein homeostasis.47 Other functions of interest include proinflammatory cytokine suppression, ion channel repair, and preservation of mitochondria, membranes, endoplasmic reticulum, and nuclei. Lastly, they also participate in intracellular transport.48,49 More specifically, Hsp70 helps misfolded proteins to adopt their native conformation and recover their function. In addition, it protects nascent proteins, promotes protein transport from the organelles, and reduces toxic aggregates.50 Hsp70 also has an important role in inflammatory and immune response signalling.51 As for oxidative stress and mitochondrial apoptosis, these can be prevented by inducing Hsp70.52 This is important since it is known that NADPH oxidases actively participate in the inflammation mechanism, since they catalyse production of O2− and other reactive oxygen species (ROS), which are widely known to be the main components responsible for cell damage.53 Moreover, a recent study showed that Hsp70 is related to insulin resistance-related vascular complications. It promotes vasodilation and inhibits thrombosis and cell proliferation by increasing angiotensin 1–7 through eNOS expression.54 Therefore, Hsp70's role is central for modulating the inflammatory process.55 It should be noted that an increase in Hsp70 would correspond to a chronic elevation in NO.56 If this is sustained over time, AKT, AMPK, and eNOS phosphorylation will increase, improving the bioavailability of endogenous NO and thus optimising the vasomotor and vasoprotective response.57 In contrast, insulin resistance and/or diabetes are associated with altered endothelial NO levels, which leads to oxidative stress-related inflammation and atherosclerosis.58,59
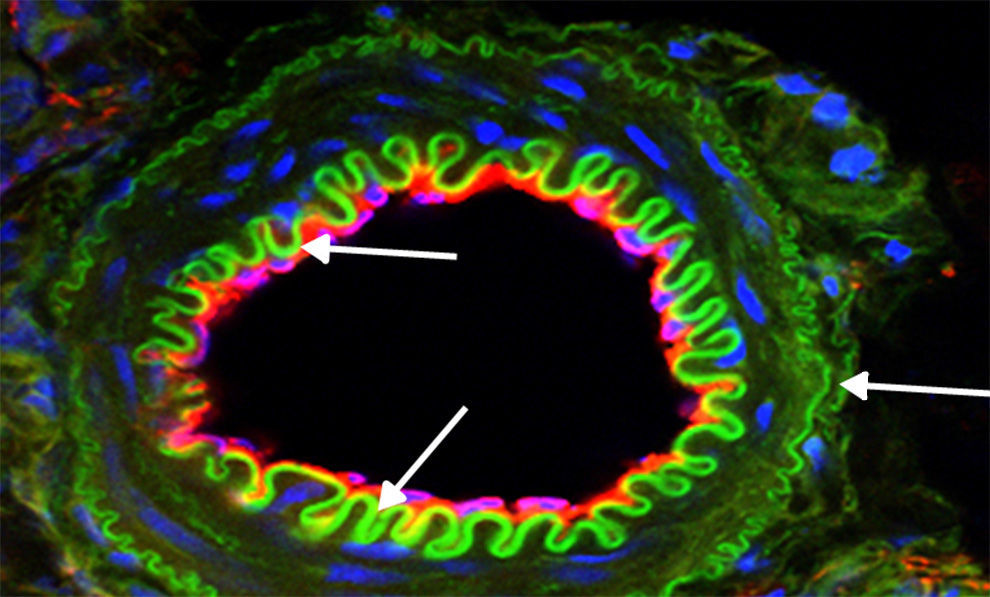
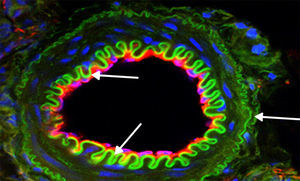
Furthermore, an original report by Xu et al.,60 drawing on the premise that HSPs are increased in vascular smooth muscle cells during acute hypertension and atherosclerosis with disturbances in NO levels, proposed that NO would induce Hsp70 expression in those cells by activating its transcription factor 1 (Hsf1). Specifically, it suggested that inducing Hsp70 could be important for protecting smooth muscle cells injured by NO overproduction. This was consistent with later studies where the pleiotropic effects of certain statins were assessed. Here the induction and nuclear translocation of Hsf1 with the consequent increase in Hsp70, eNOS, and thrombomodulin was verified.61 These results contribute to the knowledge about eNOS dysfunction as a key determining factor in atherosclerosis and that certain HSPs inhibit smooth muscle cell proliferation and participate in vascular repair.25 Moreover, a study conducted on the effects of an anti-ulcer drug known as geranylgeranylacetone (GGA) in an atherosclerosis model demonstrated iNOS suppression in vascular smooth cells previously stimulated by cytokines through NF-kappa B modulation. This effect was also associated with Hsp70 production.62 Therefore, in inflammatory states such as the atherosclerosis process (Fig. 2), modulating NO levels by inhibiting iNOS and/or inducing eNOS would lead to higher Hsp70 expression, and this would be reflected in protective effects on the cardiovascular system.
Expression of proteins of interest using immunofluorescence. Representative image: vessel coming from a kidney slice from a rat treated with l-arginine for 15 days. The higher bioavailability of NO induced a significant green expression of Hsp70 (arrows), and this was correlated with less apoptosis and reduced NADPH activity. Magnification 600×.
Although the concept of functional foods was introduced, it should be pointed out that presently there are controversies regarding drawing a line that pinpoints the specific characteristics of this food group that differentiates them from the rest. As a result, there is no definition of functional foods that is universally accepted, and therefore, there are few specific regulations in Western countries.63 This is in contrast to the East, where functional foods are regulated and marketed after approval with the identification “FOSHU” (Food for Specified Health Uses). Interestingly, the benefits attributed to these foods come from functional components or ingredients that have bioactive compounds capable of preventing different chronic conditions.
Functional foods can be animal- and/or plant-based. The latest ones stand out for their high content in active compounds, in addition to those that are considered nutrients (e.g., vitamins and minerals). In particular, they are physiologically active compounds, resulting from the secondary metabolism of plants, also called phytochemicals. Based on their chemical structures, they are grouped into: terpenes, phenols, alkaloids, and sulphur compounds, which have positive effects on human health.64 Among the phytochemicals demonstrating beneficial properties related to cardiovascular health – and reductions in cholesterol levels in particular – components such as soy protein, soluble fibre, vitamins E, C, β-carotene, phenolic compounds, and organosulphur compounds (OSC) can be mentioned.65,66 Moreover, a recent review67 has gathered evidence that describes cardiovascular protection in patients associated with the consumption of compounds such as catechins, flavonoids, and alliin (an OSC), among others. Regarding the OSC compounds, they are found in two types of cultivars: in the Brassicae and Allium genera, demonstrating similarities and differences for each of them. These OSCs have drawn attention since ancient times, with many uses proposed such as foods, condiments, and for phytotherapeutic preparations. The beneficial effects associated with consuming Allium, such as Allium sativum L. (garlic), are attributed to compounds found in the plants when the tissue is intact, odorants formed when the tissues are broken, substances derived from subsequent reactions, or even metabolic degradation products from these three types of compounds.68
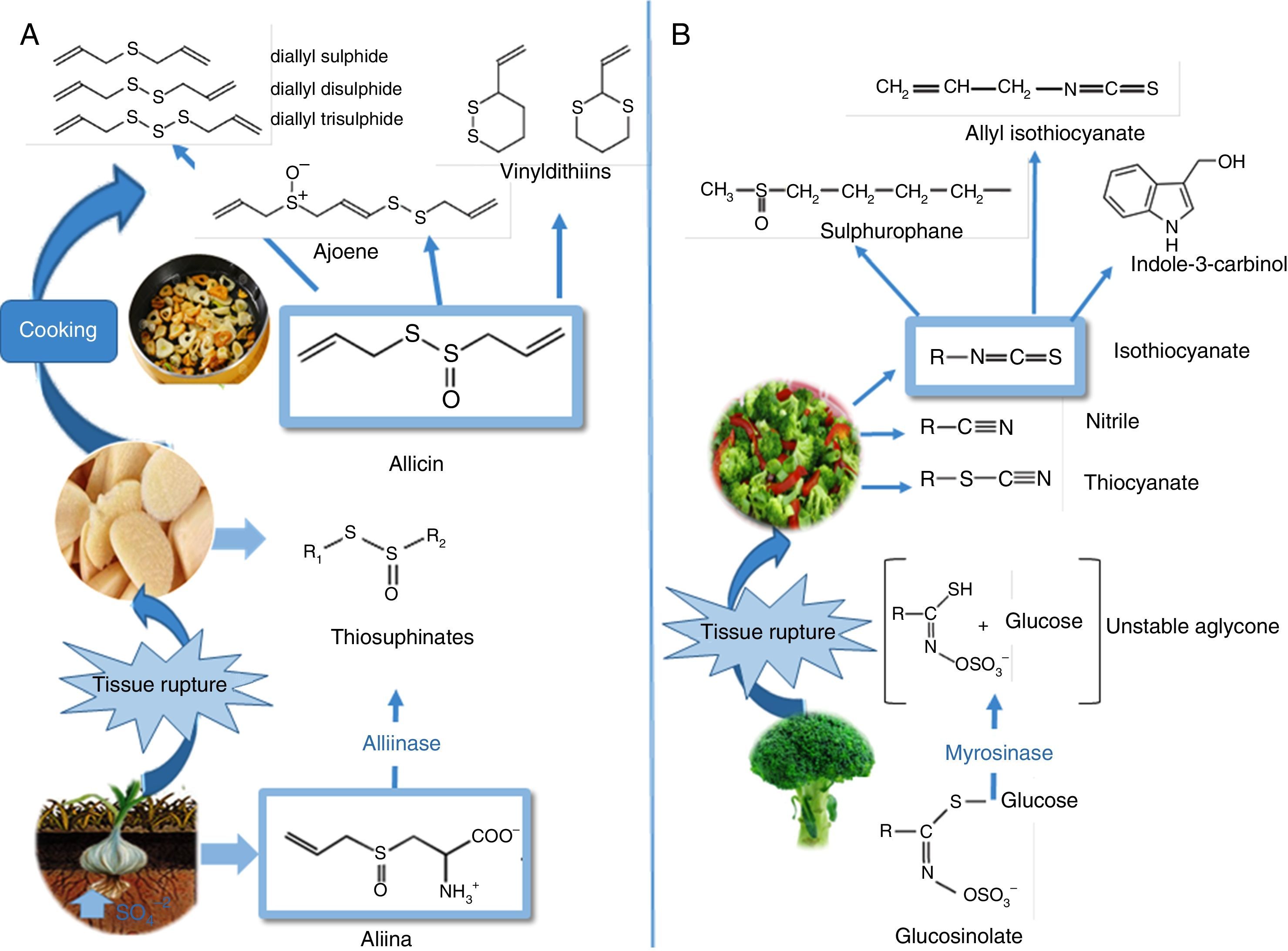
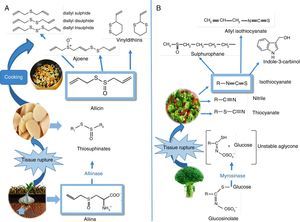
To understand the positive effects on cardiovascular health, it is worth mentioning that the biosynthesis of OSCs starts when sulphur is absorbed from the ground, it then undergoes a series of metabolic transformations to create diverse products which are stored a posteriori such as γ-glutamyl-peptides, and which are later biotransformed into alkenyl-cysteine-sulphoxides (ACSO), also called flavour precursor compounds. When the fresh garlic tissue is damaged, the alliinase enzyme (located in the vacuoles) acts on the ACSOs (present in the cell cytoplasm), releasing thiosulphinates. Allicin (diallyl thiosulphinate) is the product found in the largest proportion (65–75%) (Fig. 3A). As a result, many of the beneficial properties for cardiovascular health are attributed to this majority compound, which in turn is broken down to create another series of products such as polysulphides (mono, di, and tri-sulphides), ajoenes, and vinyldithiins, which also show protective properties. Furthermore, multiple studies report that garlic's positive effects on cardiovascular health are observed when it is eaten raw69 and/or cooked.70,71 With special focus on this review, several works have mentioned the positive effects of consuming garlic and/or its preparations on blood lipid profiles, including reducing total cholesterol, LDLc, and increasing the HDL and triglyceride levels.72–74 Meanwhile, a study conducted by Gardner et al.75 did not find any equivalent benefits in adult patients with moderate hypercholesterolaemia.
Organosulphur phytochemicals present in vegetables from the Allium sp. and Brassica sp. genera. (A) Chemical structures and transformations of the organosulphurs present in garlic (Allium sativum L). Biosynthesis of alkenyl-cysteine-sulphoxide compounds (including alliin), from sulphate absorbed by the roots. Alliinase-mediated alliin lysis and allicin production. Decomposition of allicin through cooking. (B) In Brassica, isothiocyanates formed as a result of myrosinase-mediated hydrolysis of glucosinolates The figure shows the main isothiocyanates that are formed in broccoli (Brassica oleracea italica L): sulforaphane, indol-3-carbinol, and allyl-isothiocyanate.
On the other hand, in the case of the Brassicae genus, the OSCs of biological importance originate when processing the foods and/or chewing, when the enzyme myrosinase (MS) (Fig. 3B) comes into contact with glucosinolates (GLS), hydrolysing them and creating aglycone, an unstable intermediate compound (thiohydroxamate-O-sulphonate), which is quickly converted into an extensive group of bioactive metabolites such as: isothiocyanates (ITC),76 thiocyanates, nitriles, and cyanoepithioalkanes. Regarding the ITCs, the literature describes a wide variability between the different species, and even within the same species.77
Lastly, recent evidence demonstrates that plants in the Brassicaceae family have properties related to cancer chemoprevention.78 There are also results that demonstrate inflammatory process modulation, reduced oxidative markers, increased cholesterol metabolism,79 and CVD prevention.80
Functional foods, nitric oxide, and Hsp70 in atherosclerosisThe current state of knowledge discusses multiple causes of atherosclerotic disease; however, inflammation and oxidative stress play a fundamental role in the pathogenesis of endothelial dysfunction that is commonly attributed to a change in the bioavailability of NO. In addition, Hsp70 protects cell elements from injury by reducing oxidation, inflammation, and apoptosis.81 Therefore, there is concrete evidence that suggests that NO and Hsp70 are key elements in the inflammatory and oxidative process underlying this disease. As a result, there is growing interest in diet implementation studies based on the so-called functional foods and their impact on chronic inflammatory pathologies such as atherosclerosis. In this regard, an emerging role has been suggested for the NO and Hsp70 pathways linked to the use of these special nutrients. More specifically, the use of functional and/or nutraceutical foods represents new approaches for understanding, preventing, and possibly treating CVD. In this sense, garlic and allicin are widely recognised for their therapeutic potential.23 Allicin protects by reducing oxidative stress, modulating NO, and significantly increasing Hsp70.24 This is significant since dysfunction of the enzyme eNOS is one of the main determining factors of atherosclerosis. Moreover, certain HSPs that inhibit the proliferation of smooth muscle cells participate in vascular repair.25
With regard to the NO pathways linked to the use of functional foods, prior and original works on CVD reported that garlic could prevent multiple alterations, particularly by inhibiting platelet aggregation. The studies indicated that it inhibits cyclooxygenase activity and thromboxane A2 formation, suppresses platelet Ca2+ mobilisation, and increases AMPc and GMPc levels. Furthermore, garlic also showed antioxidant properties and modulated enzyme activity from the enzyme NOS and its product, NO. Moreover garlic demonstrated the properties of a hypolipidaemic agent and improved endothelial dysfunction. The authors concluded that garlic inhibits platelet aggregation through multiple mechanisms, and suggested that it could have a role in CVD prevention.82,83 More recently, garlic has been characterised and recognised for its immense therapeutic potential with positive effects against a wide spectrum of diseases, including cancer, diabetes and microbial infections, as well as immune disorders and CVD. In this regard, an important research group has suggest that it reduces cardiovascular and metabolic risk by normalising plasma lipids and oxidated low-density lipoproteins, modulating platelet aggregation, regulating blood pressure, and preventing heart injury. These benefits would most correspond to consuming foods such as garlic, and due to direct and/or indirect mechanisms would condition hydrogen sulphide and NO generation in both cardiomyocytes and in endothelial cells.84 Moreover, studies on hypertension and the use of active garlic compounds demonstrated improvements in parameters including oxidative stress, NO bioavailability, hydrogen sulphide production, angiotensin-converting enzyme activity, NF-kappa B expression, and vascular smooth muscle cell proliferation. This review suggests that certain functional foods have significant medicinal properties with the potential to improve hypertension and the associated morbidity.85 Therefore, consistent with the inflammatory and oxidative nature of CVD, the antioxidant and anti-inflammatory effects that they demonstrate stand out among the emerging concepts on the use of functional foods and cardiovascular health. Thus, using functional foods such as garlic and its active derivatives could reduce the increase in the production of ROS, which would re-establish vascular function, improve altered vascular permeability, and reduce inflammation, accompanied by recovery of the vascular modulating function, vascular relaxation and vasoconstriction equilibrium, and reduced inflammatory adhesion molecules.86 In this sense, Lopez-Jaramillo87 has very recently discussed the role of adiponectin in CVD and he emphasises adiponectin's association with a lower risk of cardiovascular disease with an improvement in the differentiation of preadipocytes/adipocytes and an increase in endothelial NO production. Furthermore, this same author proposes and discusses how calorie restriction, moderate alcohol consumption, and the Mediterranean diet would increase adiponectin concentrations. More specifically, administering aged garlic extract and foods with pistachios could increase the concentrations of adiponectins in individuals with metabolic syndrome.
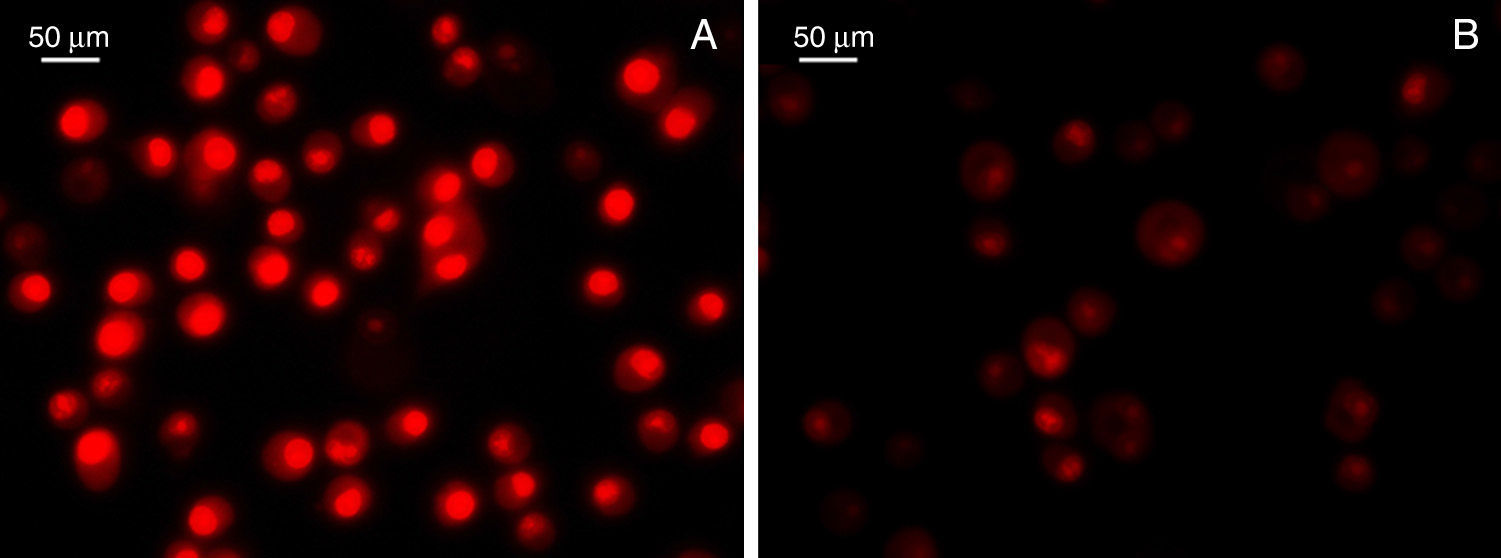
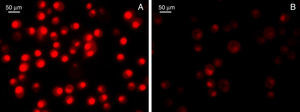
Furthermore, by following our line of reasoning, the current evidence suggests that HSPs would be involved in the immune and inflammatory mechanism during atherosclerosis development and progression. Nevertheless, the controversy of certain chaperone molecules such as Hsp60 in CVD, makes relevant Hsp70's cytoprotective properties in both basic studies17,81 and clinical studies.88,89 In addition, the association of high Hsp70 levels with low cardiovascular risk and severity was independent from the traditional risk factors.90,91 It is therefore valid to postulate that a possible hygiene, health, and/or therapeutic intervention in CVD that contributes to modulating Hsp70 expression could potentially improve its progression, or that of any chronic inflammatory disease.92 According to this postulation, a significant number of foods have been studied intensely and represent a useful alternative for preserving health. As a result, inducing Hsp70 through diet constitutes a new preventative, or even therapeutic, focus on inflammatory diseases,93 as demonstrated by the use of a water soluble OSC type compound that induced Hsp70 expression and reduced paracetamol-induced inflammatory toxicity.94 A study conducted on atherosclerosis also demonstrated that a taurine-rich diet inhibited myocardial apoptosis, and this was associated with a significant increase in myocardial Hsp70.95 These results were reinforced by other studies on biologically active garlic compounds that demonstrated protective effects by reducing oxidative stress, as evidenced by the decrease in ROS, lipid peroxidation, and preservation of antioxidant enzyme activity. These events were also directly linked to a significant Hsp70 induction and the consequent NO modulation.24 Still unpublished preliminary results from our laboratory, in collaboration with Dr. Lahera, confirmed that oxidative stress modulation associated with the use of active garlic compounds reduces inflammatory markers (Fig. 4).
Detection of superoxide anion production. Dihydroethidium fluorescent dye (DHE; Invitrogen, Grand Island, NY, USA) was used, and an intensity score for the colour red enabled superoxide anion production to be assessed in mouse BV-2 glial cell cultures injured by lipopolysaccharides in the absence (A) or presence (B) of allicin.
The current state of knowledge recognises a large number of causes of atherosclerotic disease. Nevertheless, inflammation and oxidative stress stand out as fundamental determining factors in the pathogenesis of endothelial dysfunction commonly attributed to a change in the bioavailability of NO. In addition, Hsp70 protects the cell elements from injury by reducing oxidation, inflammation, and apoptosis. Interestingly, possible therapies are constantly being reviewed. In this regard, NO, a known vasoactive messenger gas, has been closely linked to the inflammatory, oxidative, and mitochondrial dysfunction process of atherosclerosis. Furthermore, it has been demonstrated very recently that alterations in the bioavailability of NO would induce HSP expression. This mechanism could also be induced by using certain functional foods as a strategy to prevent vascular ageing as well as the development of atherosclerosis. Specifically, functional foods such as allicin would protect by reducing oxidative stress, modulating NO, and significantly increasing Hsp70. This is important since it is known that eNOS dysfunction is the main cause of atherosclerosis and that certain HSPs inhibit smooth muscle cell proliferation and participate in vascular repair. Lastly, better understanding the mechanisms involved in the development of atherosclerosis would enable us to propose new hygiene, health, and therapeutic strategies such as implementing healthy foods.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were conducted on human beings or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols of their work site regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data is contained in this paper.
Conflicts of interestThe authors declare that they have no potential conflicts of interest regarding the research, authorship, and/or publication of this review article.
Please cite this article as: Camargo AB, Manucha W. Potencial rol protector del óxido nítrico y Hsp70 asociado a alimentos funcionales en la aterosclerosis. Clin Invest Arterioscler. 2017;29:36–45.