Chronic kidney disease (CKD) is a major health problem that contributes to the development of cardiovascular disorders such as heart failure and arteriosclerotic cardiovascular disease (ACVD). The aims of this study were to determine the prevalence of CKD and to assess its association with ACVD and cardiometabolic risk factors.
MethodsCross-sectional observational study conducted in primary care setting. Population-based random sample: 6588 people between 18 and 102 years old (response rate: 66%). Crude and sex- and age-adjusted prevalence rates of CKD according to KDIGO were determined by assessing albuminuria and estimated glomerular filtration rate (eGFR) according to CKD-EPI, and their associations with cardiometabolic factors and ACVD were determined.
ResultsThe crude prevalence of CKD was 11.48% (95%CI: 10.72–12.27%), without significant difference between men (11.64% [95%CI: 10.49–12.86%]) and women (11.35% [95%CI: 10.34–12.41%]). The age- and sex-adjusted prevalence rate of CKD was 9.16% (men: 8.61%; women: 9.69%). The prevalence of low eGFR (<60 mL/min/1.73 m2) and albuminuria (≥30 mg/g) were 7.95% (95%CI: 7.30–8.61) and 5.98% (95%CI: 5.41–6.55), respectively.Hypertension, diabetes, prediabetes, increased waist-to-height ratio, heart failure, atrial fibrillation, and ACVD were independently associated with CKD (p < 0.001). Very high cardiovascular risk (CVR) according to SCORE was found in 77.51% (95%CI 74.54–80.49) of the population with CKD.
ConclusionsThe adjusted prevalence of CKD was 9.2% (low eGFR: 8.0%; albuminuria: 6.0%). Most of the patients with CKD had very high CVR. Hypertension, diabetes, prediabetes, increased waist-to-height ratio and ACVD were independently associated with CKD.
La enfermedad renal crónica (ERC) constituye un importante problema de salud que contribuye al desarrollo de alteraciones cardiovasculares como la insuficiencia cardíaca y la enfermedad cardiovascular arteriosclerótica (ECVA). Los objetivos de este estudio fueron determinar la prevalencia de ERC y evaluar su asociación con factores de riesgo cardiometabólicos y la ECVA.
MétodosEstudio observacional transversal realizado en el ámbito de atención primaria. Muestra aleatoria de base poblacional: 6.588 personas entre 18 y 102 años (tasa de respuesta: 66%). Se determinaron las tasas de prevalencia brutas y ajustadas por sexo y edad de ERC según KDIGO valorando albuminuria y filtrado glomerular estimado (FGe) según CKD-EPI, y sus asociaciones con factores cardiometabólicos y ECVA.
ResultadosLa prevalencia cruda de ERC fue 11,48% (IC95%: 10,72–12,27%), sin diferencia significativa entre hombres (11,64% [IC95%: 10,49–12,86%]) y mujeres (11,35% [IC95%: 10,34–12,41%]). La tasa de prevalencia ajustada por edad y sexo de ERC fue 9,16% (hombres: 8,61%; mujeres: 9,69%). La prevalencia del FGe reducido (<60 mL/min/1,73 m2) y de albuminuria (≥30 mg/g) fueron 7,95% (IC95%: 7,30–8,61) y 5,98% (IC95% 5,41–6,55), respectivamente. Hipertensión, diabetes, prediabetes, índice cintura-talla aumentado, insuficiencia cardíaca, fibrilación auricular y ECVA se asociaban independientemente con ERC (p < 0,001). El 77,51% (IC95%: 74,54–80,49) de la población con ERC tenía un riesgo cardiovascular (RCV) muy alto según SCORE.
ConclusionesLa prevalencia ajustada de ERC era del 9,2% (FGe reducido: 8,0%; albuminuria: 6,0%). La mayoría de los pacientes con ERC tenía RCV muy alto. Hipertensión, diabetes, prediabetes, índice cintura-talla aumentado y ECVA se asociaban independientemente con la ERC.
Chronic kidney disease (CKD) is characterised by a gradual deterioration of the body’s filtering function, removal of toxins, and volume control, which can lead to the development of cardiovascular problems such as heart failure and arteriosclerotic cardiovascular disease (ACVD)1.
CKD is a major public health problem with an increasing global burden of disease and is linked to serious health outcomes, poor quality of life, and high healthcare costs, most notably those derived from the renal replacement therapy that individuals with end-stage renal disease (ESRD) require. Therefore, there is a need to redirect the strategy toward early detection and early treatment so as to improve health outcomes and reduce the need for renal replacement therapy1–3.
In and of itself, CKD constitutes a cardiovascular risk factor (CVRF)4. Whether or not patients with CKD have other associated CVRFs, they are more likely to suffer from cardiovascular and all-cause mortality1. Death rates increase exponentially as kidney function worsens, mainly due to cardiovascular causes1. There is a strong correlation between clinical prognosis, albuminuria, and reduced estimated glomerular filtration rate (eGFR) (<60 mL/min/1.73 m2)2,5. Meta-analyses conducted by the Chronic Kidney Disease (CKD) Prognosis Consortium6,7 have documented the association of decreased eGFR and the presence of albuminuria with an increased risk of overall mortality, cardiovascular mortality, renal failure, acute renal failure, and progression of CKD in both the general population and in populations at high cardiovascular risk (CVR), irrespective of other CVRFs. People with CKD are 5–10 times more likely to die prematurely than to progress to ESRD.
Between 8–16% of the world’s population has CKD, albeit data differ significantly across countries and regions in the world3,8–10. During recent decades, the prevalence of CKD has risen in response to the growing prevalence of hypertension (HTA), obesity and diabetes (DM), as well as the increasing longevity of the population9,10. DM and HTA are the leading causes of CKD in countries with high socio-demographic indices and in most countries with low indices1. Despite the fact that it has been well established that proper management and treatment of DM, HTA, and dyslipidaemia are effective in slowing the progression of CKD11–13, the incidence of major adverse cardiovascular and renal events remains high among CKD patients.
The KDIGO (Kidney Disease: Improving Global Outcomes) conference, entitled Early Identification and Intervention in CKD14, identified strategies for early and optimal detection, screening, risk stratification, and treatment of CKD, such as encouraging healthy lifestyles and managing key CVRFs, so as to slow or delay the progression, lessen complications, and lighten the burden of disease. Participants agreed that these measures should be implemented immediately for those individuals at high risk and that, ideally, this should occur in the primary care setting.
The objectives of the SIMETAP-ERC study were to determine crude and age- and sex-adjusted prevalence rates of CKD in the adult population in accordance with KDIGO5, by quantifying albuminuria and eGFR on the basis of the Chronic Kidney Disease EPIdemiology collaboration equation (CKD-EPI)15 and to examine the associations between CKD and cardiometabolic factors and cardiovascular disease.
Material and methodsSIMETAP-ERC is a cross-sectional, observational study, authorised by the Health Service of the Community of Madrid (SERMAS), in which 121 competitively-selected family doctors participated until the required sample size was reached; these physicians belonged to 64 primary care centres (25% of the SERMAS health centres). A simple random sampling of 5.45% of the entire target population aged 18 years or older (194,073 adults) assigned to the SERMAS primary care physicians participating in the study was conducted using random number tables extracted using the Excel RANDOM.BETWEEN(lower, upper) function. Information concerning the material and methods of the SIMETAP study has been reported in detail in an earlier publication16. As per protocol, we excluded terminally ill, institutionalised, cognitively impaired, and pregnant subject, or those for whom information on biochemical variables was not available. Informed consent was obtained from all study participants, with a response rate of 65.8% and enrolment of 6588 study subjects with sufficient clinical and laboratory data to be evaluated.
The following variables were taken into account: body mass index (BMI): weight/height2 (kg/m2); overweight: BMI 25–29.9 kg/m2; obese: BMI ≥ 30 kg/m2; adiposity or body fat index CUN-BAE (Clínica Universitaria de Navarra-Body Adiposity Estimator)17 (−44.988 + [0.503 × age] + [10.689 × sex] + [3.172 × BMI] − [0, 026 × BMI2] + [0.181 × BMI × sex] − [0.2 × BMI × age] − [0.05 × BMI2 × sex] + [0.0021 × BMI2 × age]) sex (male = 0, female = 1); CUN-BAE-obesity (>25% [male]; >35% [female]); abdominal obesity: increased abdominal circumference (≥102 cm [men]; ≥88 cm [women]); waist-to-height ratio (WHI): abdominal circumference/height; increased WHI: WHI ≥ 0.55; HTA: systolic blood pressure (SBP) ≥ 140 mmHg and/ or diastolic blood pressure (DBP) ≥ 90 mmHg, or being on antihypertensive treatment; hypercholesterolaemia: total cholesterol ≥ 200 mg/dL; hypertriglyceridemia: triglycerides ≥ 150 mg/dL; cholesterol bound to high-density lipoprotein (c-HDL); low c-HDL: c-HDL < 40 mg/dL (males), < 50 mg/dL (females); cholesterol not bound to HDL (c-non-HDL); cholesterol bound to low-density lipoproteins (c-LDL); cholesterol bound to very low-density lipoproteins and remnants (c-VLDL); plasma atherogenic index: log (TG/c-HDL). Triglyceride-glucose index (TyG): Ln [TGxGPA/2]; atherogenic dyslipidaemia: HTG and low c-HDL; DM in accordance with the criteria of the American Diabetes Association (ADA)18: fasting plasma glucose (FPG) ≥ 126 mg/dL or glycated haemoglobin A1c (HbA1c) ≥ 6.5% as ascertained by standardised methods (ADA), 5% as measured by standardised methods (National Glycohemoglobin Standardization Program) according to the DCCT (Diabetes Control and Complications Trial) or determination of plasma glucose ≥ 200 mg/dL at any time or by means of an oral glucose tolerance test; prediabetes in individuals without DM based on ADA18 (GPA between 100 and 125 mg/dL or HbA1c between 5.7 and 6.4%) and according to the Spanish Diabetes Society (SED)19 (GPA between 110 and 125 mg/dL or HbA1c between 6 and 6.4%); metabolic syndrome: according to harmonised IDF/NHLBI/AHA/WHF/IAS/IASO consensus20; CVD: coronary heart disease (CHD), cerebrovascular disease (stroke), peripheral arterial disease (PAD); CHD: ischaemic heart disease, acute myocardial infarction, acute coronary syndrome, coronary revascularisation; stroke: cerebral ischaemia, intracranial haemorrhage, transient ischaemic attack; PAD: intermittent claudication, ankle-brachial index ≤0.9; CVR categories (low, moderate, high, and very high) according to SCORE21 and SCORE-OP22 for low-risk countries; eGFR according to the CKD-EPI equation15; eGFR categories as per KDIGO:5 G1 (≥90 mL/min/1.73 m2), G2 (60−89 mL/min/1.73 m2), G3a (45−59 mL/min/1.73 m2), G3b (30−44 mL/min/1.73 m2), G4 (15−29 mL/min/1.7 m2), and G5 (<15 mL/min/1.73 m2); reduced eGFR: GFR < 60 mL/min/1.73 m2; urine albumin-to-creatinine ratio (ACR) categories according to KDIGO5: A1 (<30 mg/g); A2 (30−300 mg/g); A3 (>300 mg/g); albuminuria: ACR ≥ 30 mg/g. CKD5: reduced eGFR and/ or albuminuria.
Statistical analysis was performed with the Statistical Package for the Social Sciences. Qualitative variables were analysed using percentages, chi-square test, and odds ratios (OR) with 95% confidence interval (95% CI). Continuous variables were assessed using mean with standard deviation (±SD) and Student’s t-test or analysis of variance. Medians and interquartile ranges (IQR) were determined for age and renal parameters. Crude and age- and sex-adjusted prevalence rates were determined by direct method, using standardised ten-year age groups of the Spanish population in January 2015 reported by the National Institute of Statistics23.
To assess the individual effect of comorbidities and CVRFs on the dependent variable CKD, a multivariate logistic regression analysis was carried out using the backward stepwise method, initially introducing all the variables that showed an association in the univariate analysis up to a value of p < 0.10 into the model, except for the CUN-BAE variables obesity17 and metabolic syndrome20, as these are complex variables whose defining criteria were already included in the analysis, and erectile dysfunction, as it only affects men. Subsequently, the variable that contributed least to the fit of the analysis was eliminated at each step. All tests were regarded as statistically significant if the 2-tailed p-value was less than 0.5. A bibliographic search was performed in PubMed, Medline, Embase, Google Scholar, and Web of Science to compare the CKD prevalence rates of the present study with other similar studies published since 2001.
ResultsStudy populationThe study population comprised 6588 adults between the ages of 18 and 102.8 years, with a mean (±SD) age of 55.1 (±17.5) years and a median (IQR) age of 54.69 (41.68–68.09) years. The percentage difference between men (44.1% [95%CI: 42.9–45.3 %]) and women (55.9% [95%CI: 54.7–57.1 %]) was significant (p < 0.01). The median (IQR) ages of the male and female populations were 55.0 (42.4–67.5) years and 54.5 (41.0–68.8) years, respectively, with the difference in mean [±SD] ages between men (55.3 [±16.9] years) and women (55.0 [±18.0] years) being non-significant (p = 0.634).
Chronic kidney disease prevalence ratesThe crude prevalence rate of CKD was 11.48% (95% CI: 10.72–12.27%); the difference was not significant (p = 0.711) among males (11.64% [95% CI: 10.49–12.86%]) and females (11.35% [95% CI: 10.34–12.41%]). When adjusted for age and sex, the prevalence rate of CKD was 9.16% (8.61% among men; 9.69% among women).
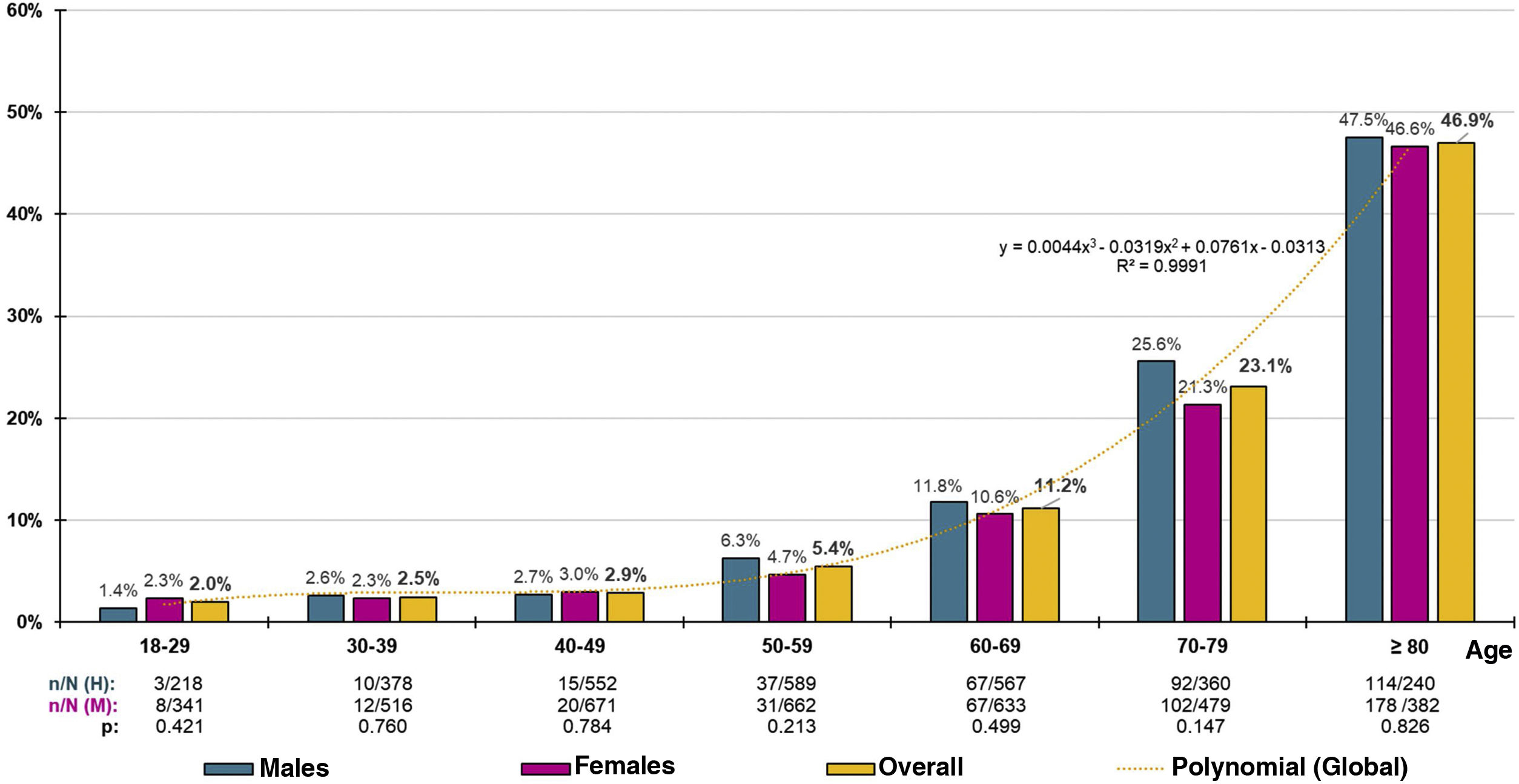
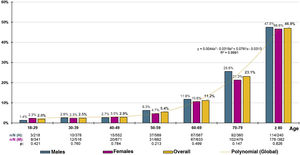
The distribution of decadal age-group-specific rates of CKD prevalence increased precisely with age (R2 = 0.999) according to the polynomial function and = 0.044x3 − 0.319x2 + 0.761x − 0.313, with no significant differences detected between sexes (Fig. 1). The age- and sex-adjusted prevalence of CKD in the ≥60-year-old population was 23.75% (23.49% in men; 24% in women), with no significant difference (p = 0.923) between the crude prevalence rates of CKD in males (23.39% [95% CI: 20.99–25.93 %]) and females (23.23% [95% CI: 21.11–25.45 %]). In the ≥70-year-old population, the age- and sex-adjusted prevalence rate of CKD was 33.56% (34.09% for men; 33.27% for women), with no significant difference (p = 0.470) between the crude prevalence rates of CKD among males (34.33% [95% CI: 30.54–38.29%]) and females (32.52% [95% CI: 29.40–35.76%]).
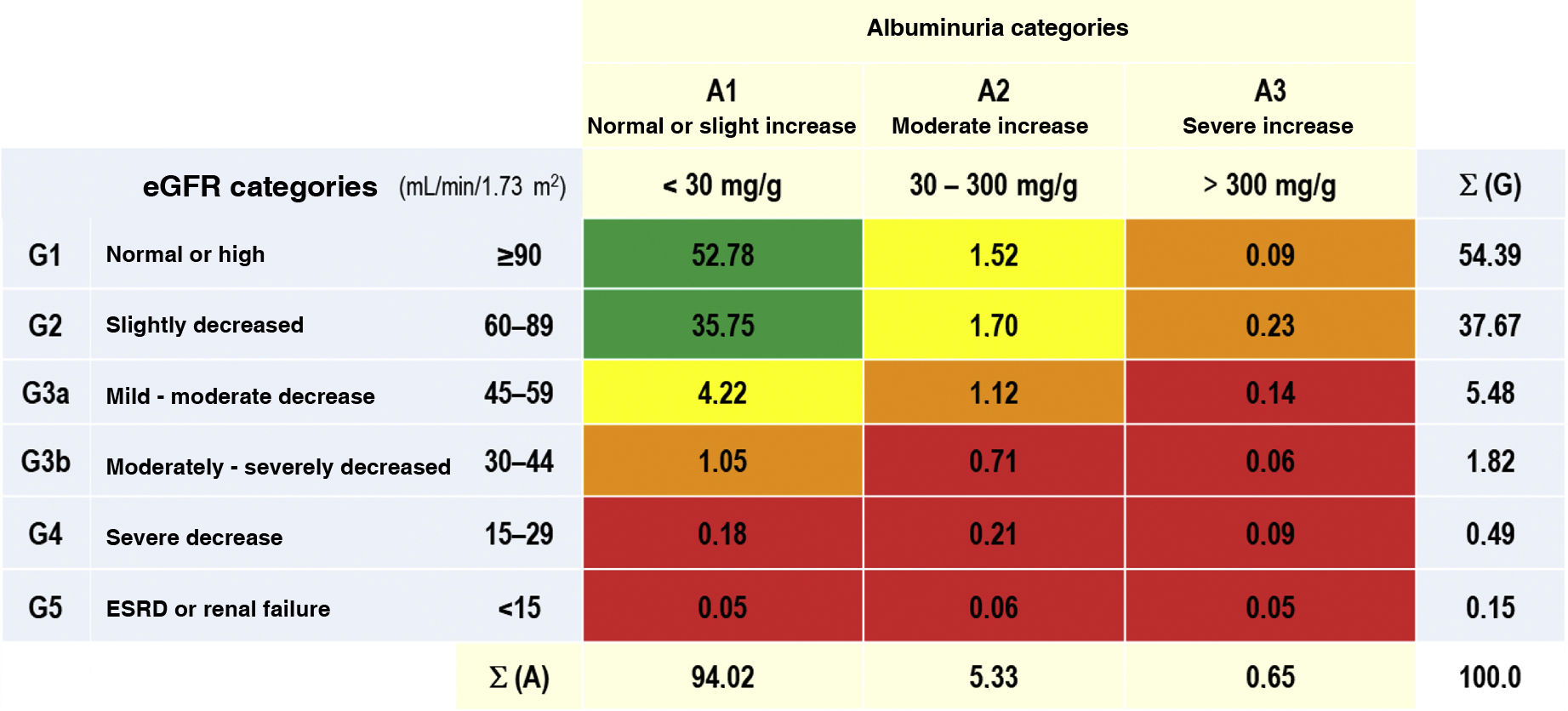
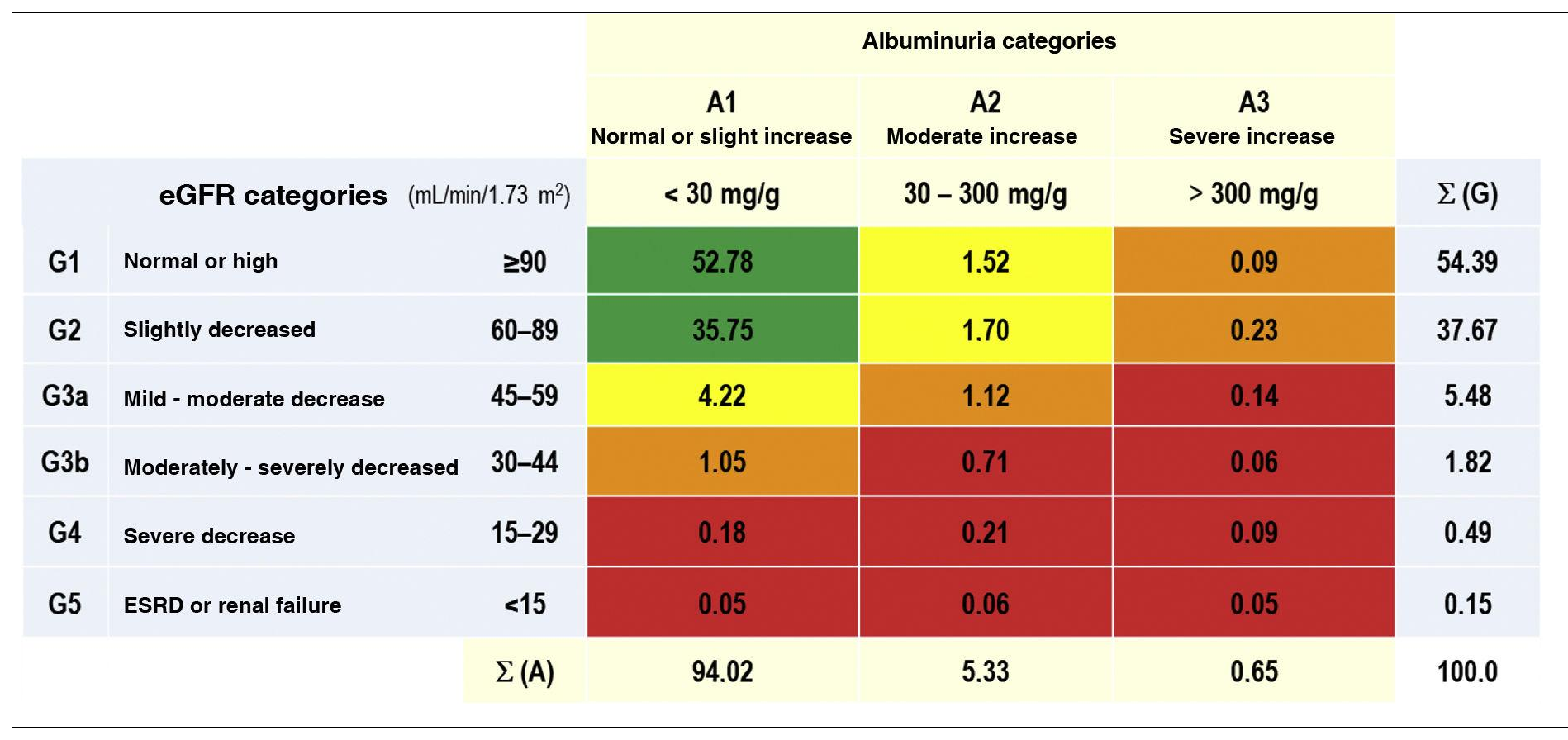
The prevalence rates of the different types of CKD depending on eGFR and albuminuria categories according to KDIGO5 were as follows: G1 and G2 with albuminuria (ACR ≥ 30 mg/g): 3.54% (95% CI: 3.09–3.98); G3a with/without albuminuria: 5.48% (95% CI: 4.93–6.03); G3b with/ without albuminuria: 1.82% (95% CI: 1.50–2.14); G4 with/ without albuminuria: 0.49% (95% CI: 0.32−0.65); G5 with/without albuminuria: 0.15% (95% CI: 0.6−0.25) (Table 1). There were no significant differences between sexes in the CKD categories according to eGFR, except in the G2 category, which was significantly higher (p = 0.12) in men (39.36% [95% CI: 37.58–41.16]) than in women (36.35% [95% CI: 34.79–37.92]). The percentage of study subjects with a lower eGFR (<60 mL/min/1.73 m2) was 7.95% (95% CI: 7.30–8.61), with no significant difference (p = 0.169) between men (7.44% [95% CI: 6.48–8.39]) and women (8.36% [95% CI: 7.47–9.25]) (Table 2).
Percentages of the categories of CKD, as per KDIGO5.
CKD: chronic kidney disease; ESRD: end-stage renal disease; eGF: estimated glomerular filtration rates according to CDK-EPI15; KDIGO5: Kidney Disease: Improving Global Outcomes; Σ (A): sum of percentages of categories of albuminuria; Σ (G): sum of percentages of categories of eGF.
KDIGO5 risk scales: green (low risk); yellow (moderately increased risk); orange (high risk); red (very high risk).
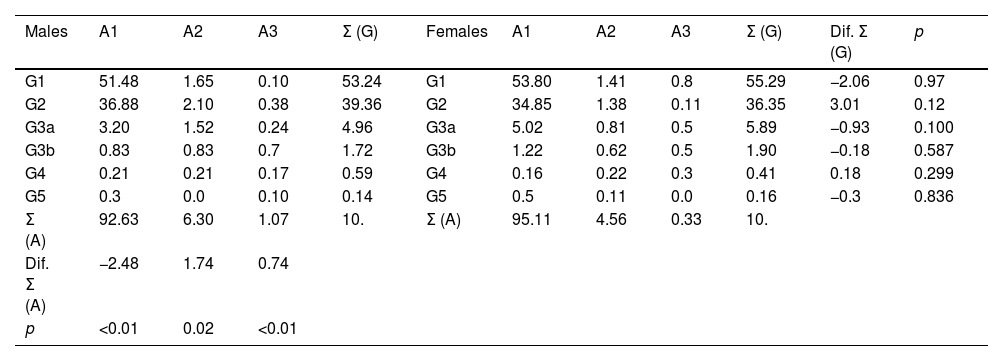
Percentages of the categories of eGF and albuminuria among males and females.
| Males | A1 | A2 | A3 | Σ (G) | Females | A1 | A2 | A3 | Σ (G) | Dif. Σ (G) | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | 51.48 | 1.65 | 0.10 | 53.24 | G1 | 53.80 | 1.41 | 0.8 | 55.29 | −2.06 | 0.97 |
| G2 | 36.88 | 2.10 | 0.38 | 39.36 | G2 | 34.85 | 1.38 | 0.11 | 36.35 | 3.01 | 0.12 |
| G3a | 3.20 | 1.52 | 0.24 | 4.96 | G3a | 5.02 | 0.81 | 0.5 | 5.89 | −0.93 | 0.100 |
| G3b | 0.83 | 0.83 | 0.7 | 1.72 | G3b | 1.22 | 0.62 | 0.5 | 1.90 | −0.18 | 0.587 |
| G4 | 0.21 | 0.21 | 0.17 | 0.59 | G4 | 0.16 | 0.22 | 0.3 | 0.41 | 0.18 | 0.299 |
| G5 | 0.3 | 0.0 | 0.10 | 0.14 | G5 | 0.5 | 0.11 | 0.0 | 0.16 | −0.3 | 0.836 |
| Σ (A) | 92.63 | 6.30 | 1.07 | 10. | Σ (A) | 95.11 | 4.56 | 0.33 | 10. | ||
| Dif. Σ (A) | −2.48 | 1.74 | 0.74 | ||||||||
| p | <0.01 | 0.02 | <0.01 |
A1: urine albumin to creatinine ratio (UACR) < 30 mg/g; A2: UACR between 30 and 300 mg/g; A3: UACR > 300 mg/g; eGF: estimated glomerular filtration rates according to CKD-EPI15; G: categories of eGF according to KDIGO5; Σ (A): sum of percentages of albuminuria categories; Σ (G): sum of percentages of eGF; Dif. Σ (A): difference of Σ (A) between males and females; Dif. Σ (G): difference of Σ (G) between males and females; p: p-value of the difference in means.
The prevalence of albuminuria (ACR ≥ 30 mg/g) in men (7.37% [95% CI: 6.42–8.32]) was significantly higher (p < 0.01) than in women (4.89% [95% CI: 4.19–5.58]). The percentage of stage A1 albuminuria was significantly higher (p < 0.01) in females than in males. The percentages of stages A2 and A3 albuminuria were significantly higher (p < 0.01) in men than in women (Table 2).
Analyses of the populations with and without CKDThe median (IQR) ages of the populations with and without CKD were 77.33 (65.22–83.38) years and 52.42 (40.33–65.18) years, respectively, with the difference in mean ages being significant (p < 0.01) (Table 3). There was no significant difference (p = 0.711) in the percentage of males and females between the two populations (Table 4). All quantitative clinical variables were significantly higher in the CKD population than in the non-CKD population, except TC, c-HDL, c-LDL, c-No-HDL, alanine aminotransferase, and eGFR concentrations, which were higher in the non-CKD population, and DBP, aspartate aminotransferase concentrations, and total cholesterol/c-HDL and c-No-HDL/c-HDL indices, the differences of which were not significant (Table 3). The median (IQR) creatinine, eGFR, and ACC of the CKD population were 1.09 (0.91–1.30) mg/dL, 55.6 (46.5–73.3) mL/min/1.73 m2, and 36.1 (5.1–100.9) mg/g, respectively. The median (IQR) creatinine, eGFR, and ACC for the population without CKD were 0.80 (0.68−0.90) mg/dL, 94.2 (82.7–106.0) mL/min/1.73 m2, and 5.3 (3.0–8.7) mg/g, respectively.
Clinical characteristics of the populations with and without CKD.
| With CKD | Without CKD | Difference in means | p | |||
|---|---|---|---|---|---|---|
| N | Mean (±SD) | N | Mean (±SD) | |||
| Age (years) | 756 | 72.69 (14.97) | 5.832 | 52.87 (16.52) | 19.82 | <0.01 |
| BMI (kg/m2) | 756 | 29.28 (5.17) | 5.832 | 27.28 (5.10) | 2.00 | <0.01 |
| Abdominal circumference (cm) | 756 | 99.49 (13.69) | 5.832 | 92.56 (13.95) | 6.93 | <0.01 |
| Waist-to-height index | 756 | 0.62 (.9) | 5.832 | 0.56 (.9) | 0.6 | <0.01 |
| Adiposity (%) | 756 | 38.79 (7.97) | 5.832 | 34.22 (8.62) | 4.57 | <0.01 |
| SBP (mmHg) | 756 | 128.35 (15.61) | 5.832 | 121.09 (15.23) | 7.26 | <0.01 |
| DBP (mmHg) | 756 | 73.75 (9.72) | 5.832 | 73.28 (9.77) | 0.47 | 0.211 |
| GPA (mg/dL)a | 756 | 109.20 (37.04) | 5.832 | 94.31 (23.61) | 14.89 | <0.01 |
| HbA1c (%)b | 687 | 6.17 (1.22) | 4.546 | 5.56 (0.81) | 0.62 | <0.01 |
| TyG Index | 756 | 8.74 (0.62) | 5.832 | 8.46 (0.60) | 0.28 | <0.01 |
| Total cholesterol (mg/dL)c | 756 | 184.47 (40.95) | 5.832 | 193.85 (39.00) | −9.38 | <0.01 |
| c-HDL (mg/dL)c | 756 | 52.62 (15.19) | 5.832 | 55.12 (14.60) | −2.50 | <0.01 |
| c-LDL (mg/dL)c | 750 | 105.09 (35.45) | 5.776 | 115.34 (34.21) | −10.25 | <0.01 |
| c-VLDL (mg/dL)c | 750 | 26.14 (13.14) | 5.776 | 22.52 (12.16) | 3.62 | <0.01 |
| c-non-HDL (mg/dL)c | 756 | 131.86 (38.37) | 5.832 | 138.72 (38.36) | −6.86 | <0.01 |
| Triglycerides (mg/dL)d | 756 | 134.69 (73.43) | 5.832 | 118.68 (84.20) | 16.01 | <0.01 |
| CT/non-HDL | 756 | 3.71 (1.09) | 5.832 | 3.72 (1.14) | −0.1 | 0.797 |
| c-non-HDL /c-HDL | 756 | 2.71 (1.09) | 5.832 | 2.72 (1.14) | −0.1 | 0.797 |
| TG/c-HDL | 756 | 2.94 (2.27) | 5.832 | 2.48 (2.58) | 0.46 | <0.01 |
| c-LDL/c-HDL | 750 | 2.12 (0.85) | 5.776 | 2.23 (0.89) | −0.11 | 0.02 |
| AIP | 756 | .1 (0.28) | 5.832 | −.8 (0.29) | 0.9 | <0.01 |
| Uric acid (mg/dL) | 740 | 5.80 (1.65) | 5.428 | 4.85 (1.42) | 0.96 | <0.01 |
| AST (U/L) | 571 | 25.35 (84.02) | 4.241 | 22.76 (34.14) | 2.59 | 0.180 |
| ALT (U/L) | 737 | 23.20 (14.20) | 5.676 | 25.09 (17.25) | −1.89 | 0.04 |
| GGT (U/L) | 713 | 41.00 (55.53) | 5.362 | 32.34 (50.10) | 8.66 | <0.01 |
| UACR (mg/g) | 756 | 91.39 (159.07) | 5.832 | 6.70 (5.14) | 84.69 | <0.01 |
| eGF (mL/min/1.73 m2) | 756 | 60.30 (23.03) | 5.832 | 94.47 (16.54) | −34.17 | <0.01 |
| Creatinine (mg/dL) | 756 | 1.19 (0.62) | 5.832 | 0.80 (0.17) | 0.39 | <0.01 |
Adiposity: body fat index CUN-BAE CUN-BAE;17 AIP: atherogenic index of plasma; ALT: alanine-aminotransferase; AST: aspartate-aminotransferase; BMI: body mass index; c-HDL: cholesterol bound to high-density lipoprotein; c-LDL: cholesterol bound to low-density lipoprotein; c-VLDL: cholesterol bound to very low-density lipoproteins and remnants; c-non-HDL: cholesterol not bound to HDL (c-non-HDL); CKD: chronic kidney disease; DBP: diastolic blood pressure; eGF: estimated glomerular filtration rate, as per CKD-EPI15; GGT: gamma-glutamyl transferase; N: sample size; p: p-value of the difference in means; SBP: systolic blood pressure; SD: standard deviation; TyG Index: Triglyceride-glucose index; UACR: urine albumen/ creatinine.
CVRF and comorbidities in the populations with and without CKD.
| With CKD n.o of cases (%)N = 756 | Without CKD, n.o of cases (%)N = 5.832 | OR | P value | |
|---|---|---|---|---|
| Male sex | 338 (44.7) | 2.566 (44.0) | 1.0 (0.9–1.2) | 0.711 |
| Smoking | 101 (13.4) | 1.325 (22.7) | 0.5 (0.4–0.7) | <0.01 |
| Lack of physical activity | 396 (52.4) | 2.683 (46.0) | 1.3 (1.1–1.5) | 0.01 |
| Overweight | 313 (41.4) | 2.203 (37.8) | 1.2 (1.0–1.4) | 0.53 |
| Obesity | 304 (40.2) | 1.529 (26.2) | 1.9 (1.6–2.2) | <0.01 |
| CUN-BAE-obesity | 709 (93.8) | 4.123 (70.7) | 6.3 (4.6–8.5) | <0.01 |
| Abdominal obesity | 474 (62.7) | 2.448 (42.0) | 2.3 (2.0–2.7) | <0.01 |
| Increased WHtR | 602 (79.6) | 3.094 (53.1) | 3.5 (2.9–4.2) | <0.01 |
| Prediabetes (SED) | 89 (11.8) | 434 (7.4) | 1.7 (1.3–2.1) | <0.01 |
| Prediabetes (ADA) | 225 (29.8) | 1.224 (21.0) | 1.6 (1.4–1.9) | <0.01 |
| Diabetes | 286 (37.8) | 749 (12.8) | 4.1 (3.5–4.9) | <0.01 |
| Hypertension | 581 (76.9) | 1.966 (33.7) | 6.5 (5.5–7.8) | <0.01 |
| Hypercholesterolemia | 586 (77.5) | 3.515 (60.3) | 1.9 (1.7–2.2) | <0.01 |
| Low c-HDL | 283 (37.4) | 1.536 (26.3) | 1.7 (1.4–2.0) | <0.01 |
| Hypertriglyceridemia | 317 (41.9) | 1.630 (27.9) | 1.9 (1.6–2.2) | <0.01 |
| Atherogenic dyslipidaemia | 173 (22.9) | 768 (13.2) | 2.0 (1.6–2.4) | <0.01 |
| Metabolic syndrome | 560 (74.1) | 2.291 (39.3) | 4.4 (3.7–5.2) | <0.01 |
| ACVD | 196 (25.9) | 419 (7.2) | 4.5 (3.7–5.5) | <0.01 |
| Coronary heart disease | 107 (14.2) | 214 (3.7) | 4.3 (3.4–5.5) | <0.01 |
| Stroke | 82 (10.8) | 168 (2.9) | 4.1 (3.1–5.4) | <0.01 |
| PAD | 61 (8.1) | 89 (1.5) | 5.7 (4.1–7.9) | <0.01 |
| Erectile dysfunctiona | 157 (46.4) | 347 (13.5) | 5.6 (4.4–7.1) | <0.01 |
| Heart failure | 102 (13.5) | 82 (1.4) | 10.9 (8.1–14.8) | <0.01 |
| Atrial fibrillation | 113 (14.9) | 137 (2.3) | 7.3 (5.6–9.5) | <0.01 |
ADA: prediabetes, according to the American Association of Diabetes18; Low c-HDL: cholesterol bound to high-density lipoprotein <40 mg/dL (males), <50 mg/dL (females); CUN-BAE-obesity17: adiposity or body fat indes (Clínica Universitaria de Navarra–Body Adiposity Estimator) >25% (males), >35% (females); Atherogenic dyslipidaemia: hypertriglyceridemia and low c-HDL; PAD: peripheral arterial disease; ACVD: atherosclerotic cardiovascular disease; CKD: chronic kidney disease; CVRF: cardiovascular risk factors; Hypercholesterolaemia: total cholesterol ≥200 mg/dL; Hypertriglyceridemia: triglycerides ≥150 mg/dL; increased WHtR: waist-to-height ratio ≥0.55; Lack of physical activity: physical activity <150 min/week; N: sample size; Obesity: body mass index ≥30 kg/m2; Abdominal obesity: abdominal circumference ≥102 cm (males), ≥88 cm (females); OR: odds ratio between both populations (95% confidence interval); SED: prediabetes according to the Spanish Society of Diabetes19; Overweight: BMI 25.0–29.9 kg/m2; Smoking: cigarette or tobacco use in the past year.
All ORs for CVRFs and comorbidities between the populations with and without CKD displayed correlations with CKD, except for current smoking status, which revealed an association with subjects without CKD, and being overweight, for which the OR was not significant (Table 4).
According to CVR assessment by SCORE21 and SCORE-OP22 for low-risk countries, 22.49% (95% CI: 19.56–25.63) of the CKD population had a high level of CVR and 77.51% (95% CI: 74.54–80.49) had a very high level of CVR. The comparison between study groups of subjects with and without CKD, the OR for high CVR was 1.7 (95% CI 1.4–2.0) and the OR for very high CVR was 10.4 (95% CI 8.7–12.4).
Multivariate analysis revealed that the CVRFs and comorbidities that were independently associated with CKD were AHT, DM, pre-diabetes according to ADA criteria, increased WHtR, heart failure, atrial fibrillation, PAD, CHD, and stroke (Table 5).
Multivariate analysis of the effect of comorbidities and CVRF on CKD.
| CKD | βa | Wald | OR Exp(β)b | pc |
|---|---|---|---|---|
| Hypertension | 1.17 (0.10) | 129.53 | 3.21 (2.63–3.93) | <0.01 |
| Diabetes | 0.95 (0.11) | 76.57 | 2.59 (2.09–3.19) | <0.01 |
| Heart failure | 1.18 (0.18) | 42.67 | 3.26 (2.29–4.65) | <0.01 |
| Atrial fibrillation | 0.95 (0.16) | 36.06 | 2.59 (1.90–3.54) | <0.01 |
| Prediabetes (ADA) | 0.56 (0.11) | 27.74 | 1.75 (1.42–2.15) | <0.01 |
| Increased WHtR | 0.49 (0.11) | 21.57 | 1.64 (1.33–2.01) | <0.01 |
| PAD | 0.66 (0.20) | 11.29 | 1.94 (1.32–2.86) | 0.01 |
| Ischaemic cardiopathy | 0.37 (0.14) | 6.44 | 1.44 (1.09–1.91) | 0.11 |
| Stroke | 0.39 (0.17) | 5.57 | 1.47 (1.07–2.04) | 0.18 |
ADA: American Association of Diabetes; CKD: chronic kidney disease; CVRF: cardiovascular risk factors; Increased WHtR: waist-to-height ratio ≥0.55; PAD: peripheral arterial disease.
The surveys conducted in high-income countries have indicated similar CKD prevalence rates (11.3% in the USA24, 12.5% in Canada25, and 11.1% in Norway26). According to the 2019 GKHA (Global Kidney Health Atlas)3 report by the International Society of Nephrology (ISN), which is based on two surveys carried out in 2017 and 2019, the average prevalence rate of CKD in 21 Western European countries was 10.1%, and in Spain, it was 9.6%. The ISN-KDDC27 study, conducted in 12 developing countries with non-random convenience sampling, whose selection bias may have overestimated prevalence, yielded a CKD prevalence rate of 14.3%, with considerable variability and even significant differences in comparison with other studies undertaken in their respective countries. While the ISN-KDDC study27 demonstrated prevalence rates in India and China of 16.8% and 29.9%, respectively, other studies28,29 performed with random sampling in these same countries have reported much lower prevalence rates (7.5% and 16.8%, respectively). This bias was also evidenced in the USA between the KEEP programme30 with actively referred study subjects and the National Health and Nutrition Examination Survey (NHANES), with different CKD prevalence rates (28.7% and 13.1%, respectively). The adjusted prevalence of the present SIMETAP-ERC study (9.2%) was somewhat lower than that of the Hill et al.9 meta-analysis (13.4%), and very much in line with that of the GBD-CKD Collaboration10 meta-analysis (9.1%); both meta-analyses exhibited substantial heterogeneity among the studies analysed. The prevalence of ESRD in the SIMETAP-ERC study (0.15%) was comparable to that of the ISN GKHA report3, which ranged from 0.1% in upper middle-income countries to 0.2% in high-income countries.
In Spain, the ENRICA study31 yielded a crude prevalence of CKD of 15.1%, similar to the 14.4% in the IBERICAN study’s primary care population cohort.32 These results differ from the EPIRCE33 study, which, adopting the Modification of Diet in Renal Diseases (MDRD)34 method to assess eGFR, yielded a CKD prevalence of 9.2%, the same as in the present study using the CKD-EPI equation15. The SIMETAP-ERC study confirmed that the prevalence of CKD increased with age, doubling for every decade after the age of 40 years (Fig. 1). Other studies performed in Spain that used the MDRD34 method to calculate the prevalence of reduced eGFR have reached disparate results, such as the EPIRCE33 study (21.4%) in the ≥ 65-year-old population and the PREV-ICTUS35 study (25.9%), as well as the study by Salvador González et al.36 (15.1%) in populations aged 60 years or older. These studies33,35,36 have demonstrated lower figures than others that have determined eGFR using the CKD-EPI equation15, such as the ENRICA study31, in which the crude prevalence of CKD was 37.3% in the population aged ≥65 years, or the present study, in which the age-adjusted prevalence rates of CKD in the ≥60 years and ≥70 years of age populations were 23.8% and 33.6%, respectively. The choice of the CKD-EPI15 equation rather than the MDRD34 method is in keeping with the recommendations of the KDIGO5 guidelines and the Spanish consensus for the detection and management of CKD37, as it is more closely related to reduced eGFR values, is more accurate for values >60 mL/min/1.73 m2, and has a greater capacity to predict overall mortality, cardiovascular mortality, or the risk of kidney failure37.
All anthropometric parameters were significantly greater in the CKD population and a correlation existed with obesity, adiposity, abdominal obesity, and increased CTI. Whilst obesity has been associated with an increased risk of CKD38, only increased CTI has displayed an independent correlation with CKD in the present study, together with other comorbidities such as AHT, DM, pre-diabetes, HF, AF, PAD, CHD, and stroke, which have also been found to be associated in other studies26,31–33,36.
Despite the decline in the risk of major adverse cardiovascular events over the last few decades as a result of better control of DM, HTA, and dyslipidaemia, the prevalence of individuals with DM2 and CKD among the adult population remains very high and the trend continues to be on the rise39, which is probably due to this better control contributing to increased longevity, and thus, more time to for CKD to develop40. The SIMETAP-DM study41 found that 27.6% of the adult population with DM had CKD, a lower prevalence than the NHANES42 survey in the USA (43.5%) and similar to that of other Spanish studies, such as the one by Fernández-Fernández et al.43 (25.3%) and PERCEDIME244 (27.9%). In the present study, 37.8% of the population with CKD had DM; 29.8% had pre-diabetes, based on ADA 28 criteria and 11.8% as per SED criteria19. Despite the fact the ORs between populations with and without CKD according to ADA18 and SED19 prediabetes were similar (1.6 and 1.7, respectively), only ADA18 prediabetes was independently associated with CKD, the same as DM, which substantiates the close relationship between alterations in glycaemic metabolism and CKD9,11.
In the present study, SBP was significantly higher (7.3 mmHg) in the CKD population than in the non-CKD cohort, and 79.6% of the CKD population had HTA; this last factor was the one that was most strongly independently associated with CKD.
The fact that all the parameters included in the CUN-BAE variables, obesity17 and metabolic syndrome20 were associated with CKD accounts for the fact that both variables demonstrated a very strong association with CKD (OR 6.3 and 4.4, respectively).
On the other hand, the SIMETAP-ERC study found that 25.9% of the CKD population had CVD (CHD: 14.2%; stroke: 10.8%; PAD: 8.1%) and that both CVD taken as a whole, and CHD, stroke, or PAD taken individually, exhibited an independent association with CKD. Approximately 55% of patients with HF and 50% of individuals with AF have some degree of renal failure and some 20% of subjects with CKD have AF45,46. In the present study, 13.5% and 14.9% of the participants with CKD had HF and AF, respectively, and both conditions were identified as independent factors closely linked to CKD. More than 77% of the CKD population had a very high CVR according to SCORE21,22, which included patients with severe CKD (eGFR<< 30 ml/min/1.73 m2), with DM and target organ damage or major CVRF (smoking, HTA, or pronounced hypercholesterolaemia), with clinical or imaging-documented ARVD, and subjects with a score ≥10. This high percentage is explained by the fact that the median age of the population is 77 years with a high frequency of CVAD (26%) and cardiometabolic factors (DM 38%; obesity: 40%; metabolic syndrome 74%; HTA 77%; hypercholesterolaemia 78%).
Limitations of the present study include the fact that it did not assess the presence of renal damage directly (renal biopsy) or indirectly through imaging tests, the inability to determine causality, the possible variability between interviewers, calibration, or the possible heterogeneity of the measurement and laboratory equipment, in addition to the fact that it did not include pregnant women, terminally ill or institutionalised patients, or those with cognitive impairment. Moreover, the cross-sectional design of the present study did not allow us to assess the persistence of albuminuria or reduced eGFR, to estimate incidence rates, or to infer causal relationships between risk factors and CKD.
The varying sampling methodologies and eGFR determinations and the different median ages of the comparison study populations might account for the different CKD prevalence rates. One strength of the present study was the large, random sampling on a populational basis that included all age groups. The SIMETAP-ERC study reveals that CKD is strongly influenced by age; therefore, age-adjusted rates are necessary to be able to compare rates with other populations. Other strengths of the present study were that it evaluated the association of CKD with numerous cardiometabolic variables, in addition to eGFR measurements according to CKD-EPI15 and UACR in the entire population.
Late referral to nephrology of ESRD patients with DM or HTA is common in Spain47. The most efficient strategy to lessen the burden of CKD and limit its progression is early detection by screening for reduced eGFR and albuminuria in individuals with DM, HTA, obesity, and ASVCD, thereby facilitating diagnosis and treatment in the early stages of CKD48–50.
The SIMETAP-ERC study indicates a progressive prevalence of CKD, especially after the age of 50, the health burden of which increases the risk of ESRD, overall mortality, and cardiovascular mortality. Evaluating the epidemiological situation of CKD is extremely important to optimise available health resources, plan interventions aimed at preventing this health problem, and reduce the burden of the disease by implementing early detection and prevention strategies that are easy to apply in the primary care setting, such as lifestyle changes and adequate control of the main risk factors associated with CKD.
ConclusionsThe age- and sex-adjusted prevalence of CKD in the adult population was 9.2% (reduced eGFR: 8%; albuminuria: 6%). The 10-year age-specific CKD prevalence rates increased with age without significant differences between men and women, doubling for every 10-year age group after the age of 40 with a prevalence rate of 24% in people aged 60 years and older and 34% after the age of 70 years.
The most frequent comorbidities associated with CKD were HTN (77%), hypercholesterolaemia (77%), metabolic syndrome (74%), abdominal obesity (63%), hypertriglyceridemia (42%), obesity (40%), DM (38%), low HDL-C (37%), pre-diabetes (30%), and CVD (26%). Variables independently associated with CKD were HTN, DM, pre-diabetes, increased CTI, heart failure, atrial fibrillation, and AVCD. The high cardiovascular burden of CKD (77% with very high CVR) in an elderly population justifies the need to implement population-based measures for early detection and optimal control of associated cardiometabolic factors.
FundingFunding for the SIMETAP Study (Grant code: 05/2010RS) was approved in accordance with the provisions of Order 472/2010, dated 16 September, of the Health Department, by which the regulatory bases and the call for grants for the year 2010 of the “Pedro Laín Entralgo” Agency for Training, Research, and Healthcare Studies of the Community of Madrid are approved, for the execution of research projects in the field of health outcomes in Primary Care.
Research ethics committeeResearch Commission of the Deputy Management of Planning and Quality.
Primary Care Management. Madrid Health Service (SERMAS).
Conflict of interestsThe authors have no conflict of interests to declare.
The effort, dedication, and collaboration of the following physicians who have participated in the SIMETAP Study Research Group are most appreciated: Abad Schilling C., Adrián Sanz M., Aguilera Reija P., Alcaraz Bethencourt A., Alonso Roca R., Álvarez Benedicto R., Arranz Martínez E., Arribas Álvaro P., Baltuille Aller M.C., Barrios Rueda E., Benito Alonso E., Berbil Bautista M.L., Blanco Canseco J.M., Caballero Ramírez N., Cabello Igual P., Cabrera Vélez R., Calderín Morales M.P., Capitán Caldas M., Casaseca Calvo T.F., Cique Herráinz J.A., Ciria de Pablo C., Chao Escuer P., Dávila Blázquez G., de la Peña Antón N., de Prado Prieto L., del Villar Redondo M.J., Delgado Rodríguez S., Díez Pérez M.C., Durán Tejada M.R., Escamilla Guijarro N., Escrivá Ferrairó R.A., Fernández Vicente T., Fernández-Pacheco Vila D., Frías Vargas M.J., García Álvarez J.C., García Fernández M.E., García García Alcañiz M.P., García Granado M.D., García Pliego R.A., García Redondo M.R., García Villasur M.P., Gómez Díaz E., Gómez Fernández O., González Escobar P., González-Posada Delgado J.A., Gutiérrez Sánchez I., Hernández Beltrán M.I., Hernández de Luna M.C., Hernández López R.M., Hidalgo Calleja Y., Holgado Catalán M.S., Hombrados Gonzalo M.P., Hueso Quesada R., Ibarra Sánchez A.M., Iglesias Quintana J.R., Íscar Valenzuela I., Iturmendi Martínez N., Javierre Miranda A.P., López Uriarte B., Lorenzo Borda M.S., Luna Ramírez S., Macho del Barrio A.I., Magán Tapia P., Marañón Henrich N., Mariño Suárez J.E., Martín Calle M.C., Martín Fernández A.I., Martínez Cid de Rivera E., Martínez Irazusta J., Migueláñez Valero A., Minguela Puras M.E., Montero Costa A., Mora Casado C., Morales Cobos L.E., Morales Chico M.R., Moreno Fernández J.C., Moreno Muñoz M.S., Palacios Martínez D., Pascual Val T., Pérez Fernández M., Pérez Muñoz R., Plata Barajas M.T., Pleite Raposo R., Prieto Marcos M., Quintana Gómez J.L., Redondo de Pedro S., Redondo Sánchez M., Reguillo Díaz J., Remón Pérez B., Revilla Pascual E., Rey López A.M., Ribot Catalá C., Rico Pérez M.R., Rivera Teijido M., Rodríguez Cabanillas R., Rodríguez de Cossío A., Rodríguez de Mingo E., Rodríguez Rodríguez A.O., Rosillo González A., Rubio Villar M., Ruiz Díaz L., Ruiz García A., Sánchez Calso A., Sánchez Herráiz M., Sánchez Ramos M.C., Sanchidrián Fernández P.L., Sandín de Vega E., Sanz Pozo B., Sanz Velasco C., Sarriá Sánchez M.T., Simonaggio Stancampiano P., Tello Meco I., Vargas-Machuca Cabañero C., Velazco Zumarrán J.L., Vieira Pascual M.C., Zafra Urango C., Zamora Gómez M.M., Zarzuelo Martín N.