Vascular smooth muscle cells (VSMCs) undergo a phenotypic-switching process during the generation of unstable atheroma plaques. In this investigation, the potential implication of the tumor necrosis factor superfamily (TNFSF) ligands, in the gene expression signature associated with VSMC plasticity was studied.
Material and methodsHuman aortic (ha)VSMCs were obtained commercially and treated with the cytokine TNFSF14, also called LIGHT, the lymphotoxin alpha (LTα), the heterotrimer LTα1β2 or with vehicle for 72h. The effect of the different treatments on gene expression was analyzed by quantitative PCR and included the study of genes associated with myofibroblast-like cell function, osteochondrogenesis, pluripotency, lymphorganogenesis and macrophage-like cell function.
ResultsHaVSMCs displayed a change in myofibroblast-like cell genes which consisted in reduced COL1A1 and TGFB1 mRNA levels when treated with LTα or LIGHT and with augmented MMP9 expression levels when treated with LTα. LTα and LIGHT treatments also diminished the expression of genes associated with osteochondrogenesis and pluripotency SOX9, CKIT, and KLF4. By contrary, all the above genes were no affected by the treatment with the trimer LTα1β2. In addition, haVSMC treatment with LTα, LTα1β2 and LIGHT altered lymphorganogenic cytokine gene expression which consisted of augmented CCL20 and CCL21 mRNA levels by LTα and a reduction in the gene expression of CCL21 and CXCL13 by LIGHT and LTα1β2 respectively. Neither, LTα or LIGHT or LTα1β2 treatments affected the expression of macrophage-like cell markers in haVSMC.
ConclusionsAltogether, indicates that the TNFSF ligands through their interconnected network of signaling, are important in the preservation of VSMC identity against the acquisition of a genetic expression signature compatible with functional cellular plasticity.
La transición de placa de ateroma estable a placa inestable implica, entre otros procesos, un cambio fenotípico de las células del músculo liso vascular (CMLVs). En esta investigación, se estudió el posible papel de los ligandos de la superfamilia del factor de necrosis tumoral (TNFSF), en los cambios de expresión génica asociada a la plasticidad de las CMLVs.
Materiales y métodosLas CMLVs de aorta humana (CMLVah) se obtuvieron comercialmente y se trataron con la citoquina TNFSF14, también llamada LIGHT, la linfotoxina alfa (LTα), el heterotrímero LTα1β2 o con vehículo durante 72 horas. El efecto de los diferentes tratamientos se analizó mediante el estudio de la expresión génica por PCR cuantitativa e incluyó genes asociados con fenotipo miofibroblástico, osteocondrogénico, genes de pluripotencia, genes de linforganogénesis y genes característicos de macrófagos.
ResultadosEl estudio de genes asociados a fenotipo miofibroblástico en las CMLVah reveló una reducción de la expresión génica de COL1A1 y TGFB1 tras el tratamiento con LTα o LIGHT mientras que el tratamiento con LTα aumentó los niveles de mRNA de MMP9. LTα y LIGHT también disminuyeron la expresión de genes de osteocondrogénesis y pluripotencia como SOX9, CKIT y KLF4. Por el contrario, la expresión de los genes anteriores no se vio afectada por el tratamiento con el trímero LTα1β2. El tratamiento de las CMLVah con LTα, LTα1β2 y LIGHT alteró la expresión génica de citoquinas linforganogénicas con una expresión aumentada de los genes CCL20 y CCL21 por LTα y una reducción de los niveles de mRNA de CCL21 y CXCL13 por LIGHT y LTα1β2, respectivamente. Ninguno de los tres tratamientos alteró la expresión de genes típicos de macrófagos en las CMLVah.
ConclusionesLa presente investigación indica que los ligandos de la familia de los TNFSF a través de su red de señalización, son importantes en la preservación de la identidad de las CMLVs frente a la adquisición de una expresión génica compatible con una mayor plasticidad celular funcional.
Cardiovascular disease (CVD) is a clinical manifestation of the atherosclerotic process, currently considered both a metabolic and a chronic inflammatory disease with an active participation of the innate and adaptive immune system.1 Atherosclerosis lesion represents a profound vascular vessel wall remodeling. The lesion initiates with an endothelial dysfunction which progressively leads to the formation of fibroatheromas with an inflammatory core2 containing lipid-loaded macrophages and different T helper (Th) and regulatory T (Treg) cells.3 During the progression of the disease, vascular smooth muscle cells (VSMCs) become migratory and proliferative and undergo a phenotypic-switching process by acquiring characteristics of different cell-types such as myofibroblasts, mesenchymatic cells, macrophages, lymphoid organ organizer cells or osteochondrogenic cells.4 Studies have shown an important role of the cross-talk between the plaque-stress factors and the functional plasticity of VSMC in atheroma instability and acute coronary syndromes.5 Therefore, it is of relevance to understand potential inflammatory mediators that might affect VSMC lesional heterogeneity.
The lymphotoxins (LT) and the tumor necrosis factor superfamily 14 (TNFSF14), also called LIGHT, belong to the TNFSF and constitute with their receptors an important interconnected network signaling in immune homeostasis.6,7 LIGHT is mainly produced by immune cells and mediates its effects through two receptors, the LTβR and the herpes virus entry mediator (HVEM) while its activity is inhibited by the Decoy receptor 3 (DcR3). LIGHT-signaling through LTβR, a receptor mostly expressed in stromal and epithelial cells, activates both canonical and non-canonical nuclear factor kappa B (NFkB) pathways, exerting important functions in immune response and lymphorganogenesis.6,7 Through HVEM signaling, a receptor characteristic of T and B cells, LIGHT promotes non-canonical NFKB and c-Jun N-terminal Kinase (JNK) pathways increasing cytokine production, cell survival, and proliferation.8 The LIGHT/LTβR-HVEM axis becomes more complex due to interactions with the other LT. Thus, LTα exerts cytotoxic effects and forms the homotrimer LTα3 that binds to TNF receptor 1 (TNFR1) and TNFR2. The homotrimer LTα3 can also bind, with low affinity, to HVEM.6 In addition, LTα and LTβ form the LTα1β2 heterotrimer which is produced by lymphocytic cells, and is essential, through the LTβR/NFKB non-canonical pathway, in lymphoid tissue organogenesis during development.7 In adulthood, the LTα1β2/LTβR/NFKB interaction is important in immune responses against pathogenic insults. Hence LIGHT, LTα3 and LTα1β2 compete for the same receptors and therefore their actions will depend on their relative abundance in the circulation and within tissues.
It is not therefore surprising that the study of LIGHT and LT signaling through the LTβR/HVEM-dependent pathways in metabolic diseases have yielded discrepant results. Thus, hepatic T cell production of LIGHT in mouse models induces hypercholesterolemia by modulating hepatic enzymes9 and Light gene inactivation alleviates insulin resistance, steatosis and hepatic inflammation.10 On the other hand, T60N variant of LYMPHOTOXIN ALPHA gene has been associated with type 2 diabetes and other features of the metabolic syndrome.11 However, its deficiency does not affect obesity or insulin resistance in mouse models.12
In CVD, discrepant results have been reported as well. LIGHT levels are elevated in coronary disease,13 clinical heart failure14 and unstable angina,15 while a soluble form of LTβR has been observed in human atherosclerosis.16 In the atherosclerotic Apolipoprotein e-deficient (Apoe-/-) mice, macrophage specific deletion of Ltβr reduced atherosclerosis by augmenting the proresolving Ly6Clow monocytes.17 Consistently, soluble LIGHT acute treatment enhanced proliferant Ly6Chi-monocytes and aggravated atherosclerosis.18 Notwithstanding, in another study, Ltβr specific deletion in VSMCs in Apoe-/- mice accelerated atherosclerosis indicating atheroprotection in a LTβR-dependent manner which was attributed to T-cell homeostasis induced by a proper functionality of the artery lymphoid organs.19 Therefore, these studies unveiled a complex role of the TNFSF ligands in atherosclerosis. Notably, genetic inactivation of Light aggravated abdominal aneurysm vascular lesions.20 Specifically, in vivo and in vitro data indicated that LIGHT/LTβR-signaling disruption provoked dysregulated SOX9, OPN and BMP2 gene expression compatible with an osteochondrogenic phenotype which has been previously associated with vascular dysfunction.21 These results suggest a protective function of LIGHT/LTβR-signaling in vascular injury through the prevention of osteochondrogenic phenotype acquisition.
Given the multiple interactions and connections between the TNFSF ligands and their receptors, in the present investigation we sought to investigate the potential role of the LTα, LIGHT and the LTα1β2 heterotrimer in the VSMC phenotype.
Materials and methodsHuman aortic VSMC cell culture experimentsHuman aortic (ha)VSMCs were commercially obtained (Invitrogen, C-007-5C, Thermofisher Scientific, Madrid, Spain) and cultured in 231 medium, 20% FBS, 5% of smooth muscle growth factor (Invitrogen, S00725 Thermofisher Scientific) and 2% P/S/A (Lonza, Basel, Switzerland) as described.20,22 HaVSMCs were kept on a humidified 5% CO2 atmosphere until treatments and were used until passage 6–7. For expression experiments haVSMC were grown in 20% FBS/DMEM-P/S/A medium on 6-well plates up to 70–80% of confluency and then treated for 72h in 0.5% FBS/DMEM-P/S/A medium with vehicle, human soluble LIGHT (20ng/ml, Preprotech, Germany, Hamburg), LTα (at 5 and 10ng/ml Preprotech, Germany, Hamburg) or with LTα1β2 (at 20 and 100ng/ml, R&D systems Biotechne, Minneapolis, Minnesota, USA). For protein analysis of the receptors cells were grown as before until confluency and then treated for 24h in 0.5% FBS/DMEM-P/S/A medium with vehicle or human soluble LIGHT (50ng/ml). After treatments, cells were rinsed with PBS 1×, collected by centrifugation (500×g, 10min) snap-frozen with liquid N2 and stored for gene expression analysis by qPCR.
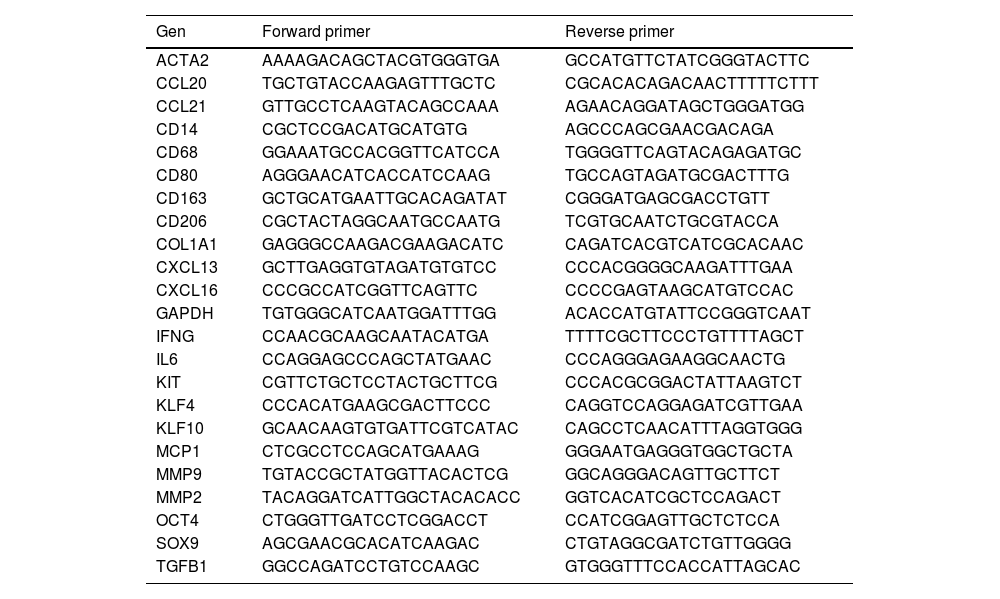
Gene expression analysis by quantitative real-time PCR (qPCR)Total RNA was obtained from cultured haVSMCs treated as indicated above using TRIzol reagent following manufacturer recomendations (Invitrogen, Carlsbad, CA, USA). A total of 500ng of RNA were reverse transcribed with the Maxima First-Strand kit (Fermentas, Waltham, MA, USA). The genes of interest were amplified with Luminaris Color HiGreen High ROX qPCR Master Mix (Fermentas, Waltham, MA, USA) on a 7900 FastSystem thermal cycler and results were analyzed with the formula 2−ΔΔCt. mRNA levels were normalized to the GAPDH mRNA levels and relativized to vehicle-treated cells. The primer sequences were obtained from the PrimerBank data base (Massachusetts General Hospital, Harvard University) and are listed in Table 1.
Sequences of primers used for qPCR expression studies.
| Gen | Forward primer | Reverse primer |
|---|---|---|
| ACTA2 | AAAAGACAGCTACGTGGGTGA | GCCATGTTCTATCGGGTACTTC |
| CCL20 | TGCTGTACCAAGAGTTTGCTC | CGCACACAGACAACTTTTTCTTT |
| CCL21 | GTTGCCTCAAGTACAGCCAAA | AGAACAGGATAGCTGGGATGG |
| CD14 | CGCTCCGACATGCATGTG | AGCCCAGCGAACGACAGA |
| CD68 | GGAAATGCCACGGTTCATCCA | TGGGGTTCAGTACAGAGATGC |
| CD80 | AGGGAACATCACCATCCAAG | TGCCAGTAGATGCGACTTTG |
| CD163 | GCTGCATGAATTGCACAGATAT | CGGGATGAGCGACCTGTT |
| CD206 | CGCTACTAGGCAATGCCAATG | TCGTGCAATCTGCGTACCA |
| COL1A1 | GAGGGCCAAGACGAAGACATC | CAGATCACGTCATCGCACAAC |
| CXCL13 | GCTTGAGGTGTAGATGTGTCC | CCCACGGGGCAAGATTTGAA |
| CXCL16 | CCCGCCATCGGTTCAGTTC | CCCCGAGTAAGCATGTCCAC |
| GAPDH | TGTGGGCATCAATGGATTTGG | ACACCATGTATTCCGGGTCAAT |
| IFNG | CCAACGCAAGCAATACATGA | TTTTCGCTTCCCTGTTTTAGCT |
| IL6 | CCAGGAGCCCAGCTATGAAC | CCCAGGGAGAAGGCAACTG |
| KIT | CGTTCTGCTCCTACTGCTTCG | CCCACGCGGACTATTAAGTCT |
| KLF4 | CCCACATGAAGCGACTTCCC | CAGGTCCAGGAGATCGTTGAA |
| KLF10 | GCAACAAGTGTGATTCGTCATAC | CAGCCTCAACATTTAGGTGGG |
| MCP1 | CTCGCCTCCAGCATGAAAG | GGGAATGAGGGTGGCTGCTA |
| MMP9 | TGTACCGCTATGGTTACACTCG | GGCAGGGACAGTTGCTTCT |
| MMP2 | TACAGGATCATTGGCTACACACC | GGTCACATCGCTCCAGACT |
| OCT4 | CTGGGTTGATCCTCGGACCT | CCATCGGAGTTGCTCTCCA |
| SOX9 | AGCGAACGCACATCAAGAC | CTGTAGGCGATCTGTTGGGG |
| TGFB1 | GGCCAGATCCTGTCCAAGC | GTGGGTTTCCACCATTAGCAC |
Protein extracts were obtained by homogenization of haVSMCs in the presence of ice-cold lysis TNG buffer (Tris–HCl 50mM, pH 7.5, NaCl 200mM, Tween-20 1% vol/vol, NP-40 0.2% vol/vol) supplemented with Complete Mini cocktail, PhosSTOP (Roche, Mannheim, Germany), beta-glycerophosphate 50mM (Sigma), 2mM phenylmethylsulfonyl fluoride (PMSF, Roche) and 200μM Na3VO4 (Sigma). For protein analysis, protein extracts (25–50μg) were prepared with Laemmli buffer (5min 95°C) and subjected to 12% w/v polyacrylamide gel electrophoresis and western blot as described.10 The following primary (1/200) and secondary (1/2000) antibodies were used to detect the proteins: HVEM (PA5-20237, ThermoFisher), LTβR (ab70063, Abcam) and β-actin (Sigma), anti-mouse IgG-HRP (P0447, Dako) and goat anti-rabbit IgG-HRP (P0448, Dako). The immunocomplexes were detected with an ECL Plus detection kit (ThermoFisher). All antibodies for western blot were acquired and used after checking that validation was performed by the manufacturer company.
Statistical analysisQuantitative data are presented as the mean±the standard error of the mean (SEM) and with the single data points. All samples were randomly treated and analyzed by observers blinded to treatments. Treatments and collection of data were obtained at the same time and order to avoid confounding effects. The exclusion criteria were applied when data was out of range of the standard curve in each experiment, when samples were lost during the experimentation and when the (non-iterative) Grubbs test identified outliers. Statistical tests were applied after the determination of normal distribution (Shapiro–Wilk and D’Agostino–Pearson normality tests) and equality of variances (F test). Differences were evaluated with unpaired Student's t test, Mann–Whitney U test (for nonparametric distribution) and one-way ANOVA followed by Bonferroni multiple comparison test (more than two groups). All statistical tests were run in GraphPad Prism 9.0.0 (GraphPad Prism Software, La Jolla, CA, USA). Differences were considered statistically significant when p-values were below 0.05: *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001.
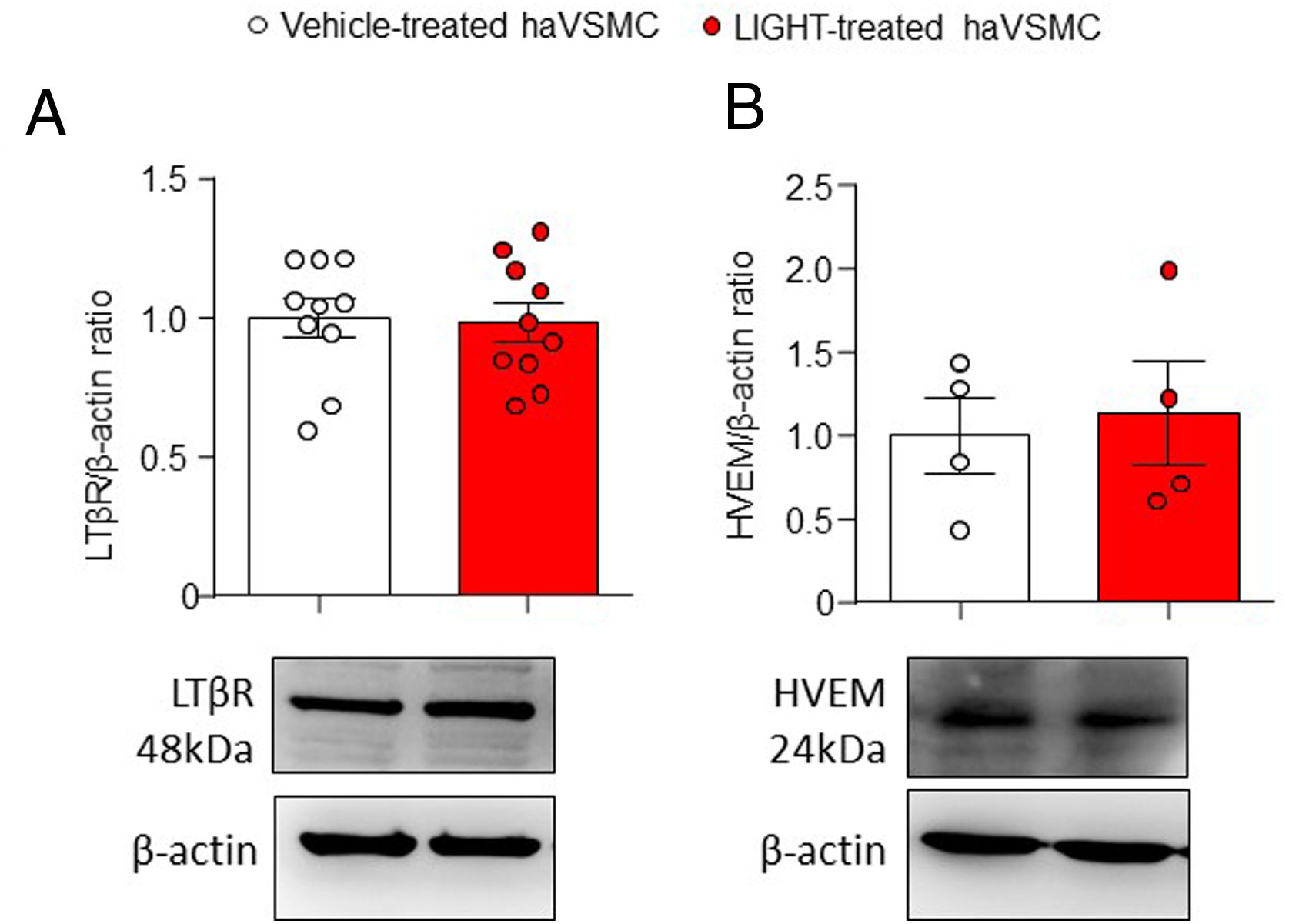
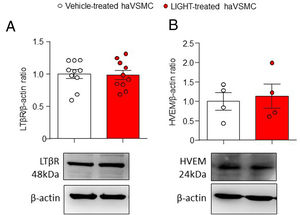
ResultsAortic human vascular smooth muscle cells express LTβR and HVEMLTβR is expressed mainly in stromal and epithelial cells while HVEM is mainly found in immune cells. However, the expression of LTβR and HVEM in liver and adipose tissue10 and the expression of LTBR gene in haVSMC20 has been previously described. To assess whether the TNFSF ligands can signal in haVSMCs protein analysis was performed. Analysis of both LTβR and HVEM receptors in vehicle- and LIGHT-treated haVSMC indicated the presence of both receptors in these cells with similar levels regardless of the treatment (Fig. 1A, B).
Analysis of the LTβR and HVEM protein content in haVSMC. Protein quantification in the western blot analysis displayed as (A) LTβR/β-actin and (B) HVEM/β-actin ratios in haVSMC treated with vehicle or LIGHT 20ng/μl overnight. Representative blots are shown for the western blot analysis. Statistical analysis were performed by the Student's t-test.
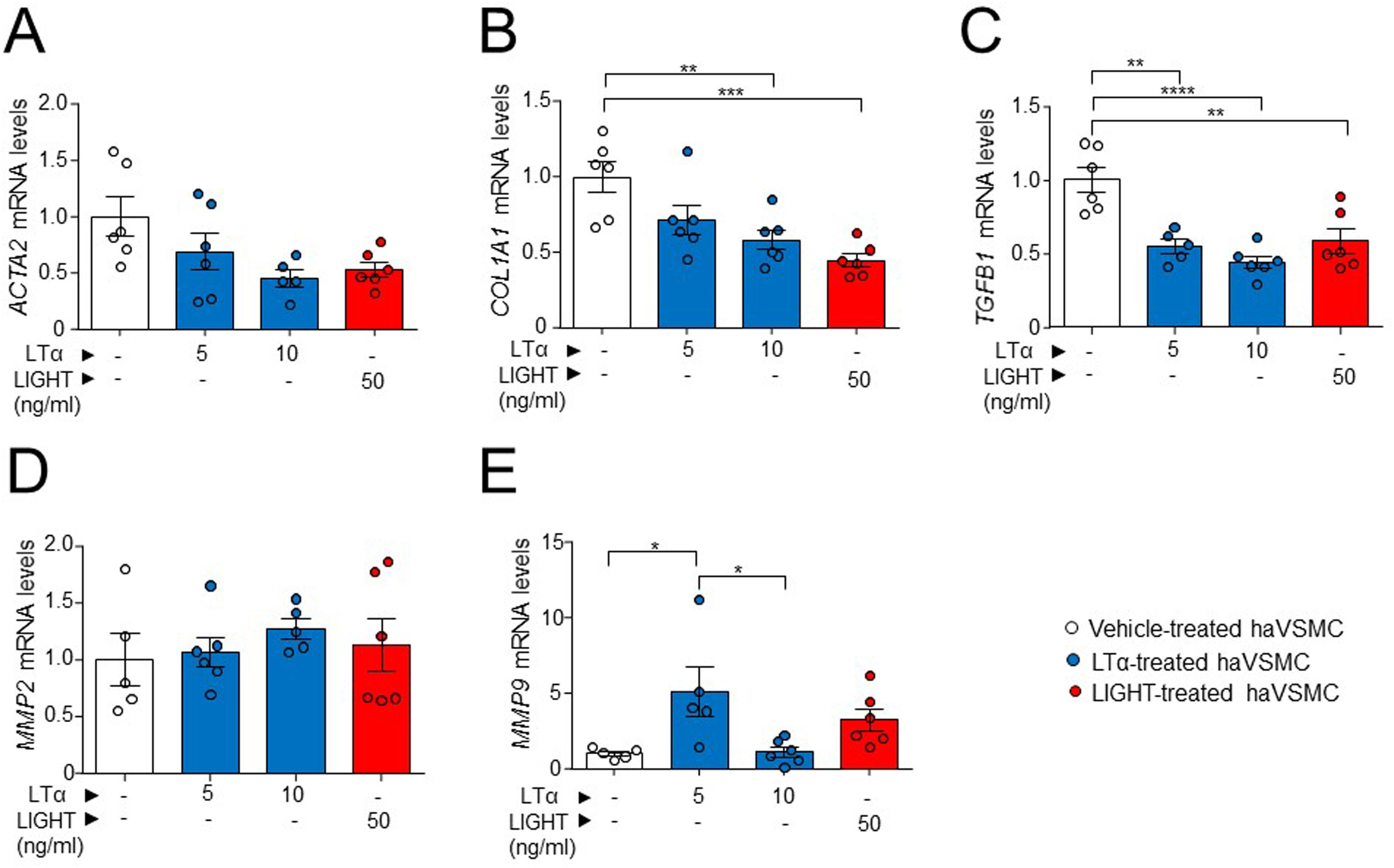
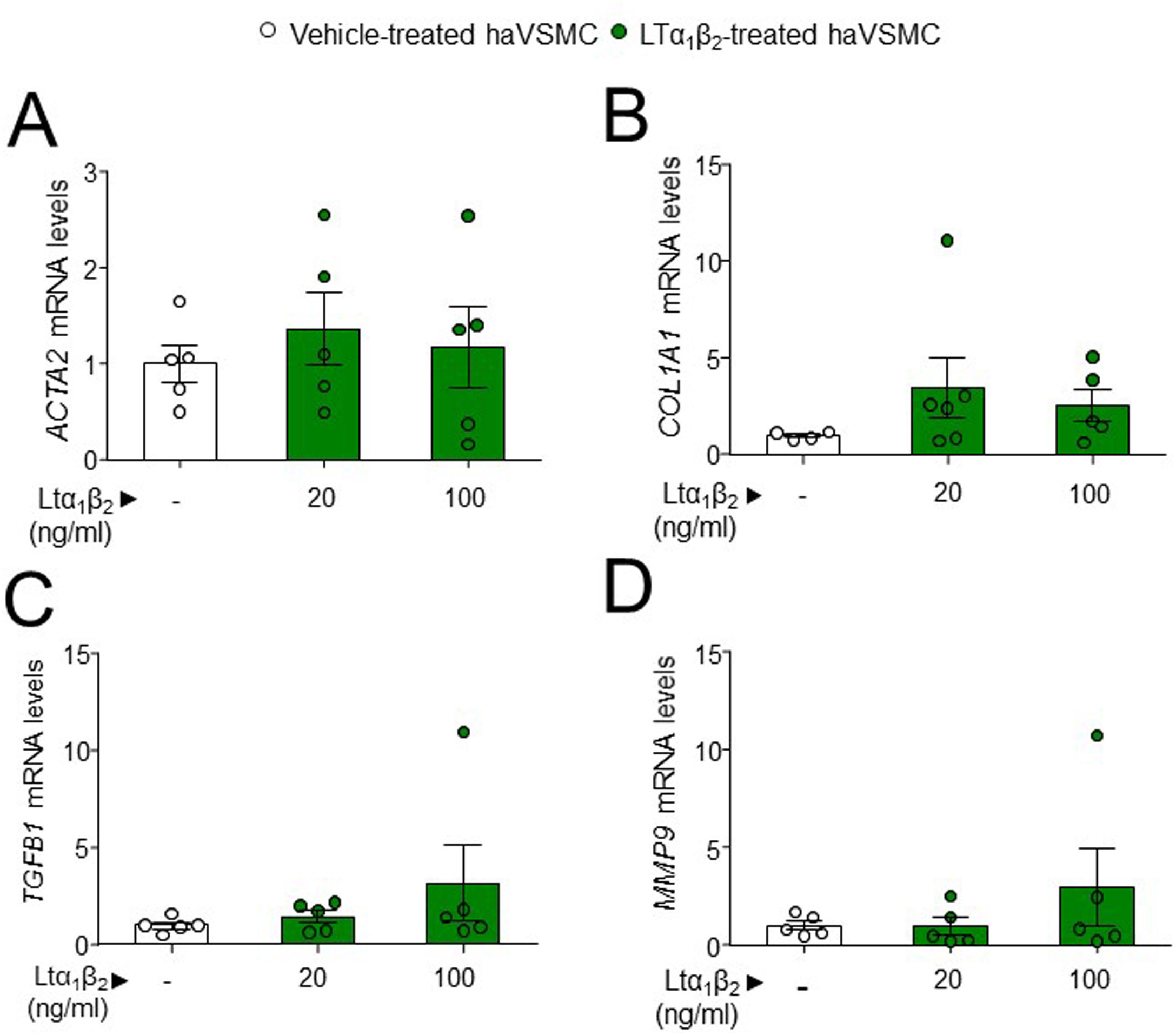
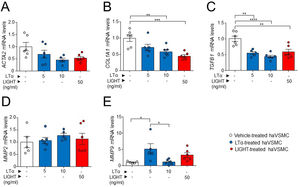
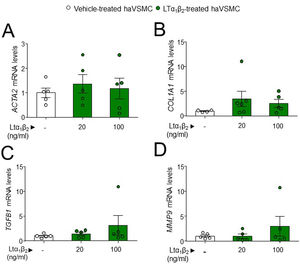
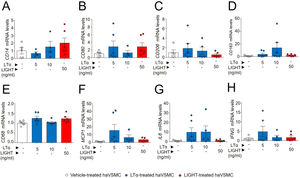
The potential effect of the LTs and LIGHT in haVSMC phenotype switching was explored by using the expression of different markers for myofibroblast-like cell. Analysis of myofibroblast-like cell genes showed reduced expression of COL1A1 and TGFB1 mRNA levels in haVSMCs treated with LTα at 10ng/ml compared with vehicle-treated cells (Fig. 2B, C). HaVSMCs treated with LTα at a lower dose of 5ng/ml also displayed reduced mRNA levels of TGFB1 and enhanced expression of MMP9 compared with vehicle-treated haVSMCs (Fig. 2C, E). No changes were observed in the expression of ACTA2 or in MMP2 genes (Fig. 1A, D). Likewise, haVSMCs treated with LIGHT displayed diminished COL1A1 and TGFB1 mRNA gene expression (Fig. 2B, C) with no changes in ACTA2, MMP2 or MMP9 (Fig. 2A, D, E). Treatment of haVSMCs with the trimer LTα1β2 did not affect ACTA2, COL1A1, TGFB1 and MMP9 mRNA levels at any of the two doses tested (20 or 100ng/ml) (Fig. 3A–D).
Expression analysis of genes related to the acquisition of haVSMC contractile and secretory phenotype. Relative expression levels of (A) ACTA2, (B) COL1A1, (C) TGFB1, (D) MMP2 and (E) MMP9 in ahVSMC treated with vehicle, LTα (5ng/ml), LTα (10ng/ml) and LIGHT (50ng/ml). mRNA levels were normalized with the endogenous gene levels and relativized to the vehicle-treated VSMC mRNA levels. Statistical analysis was performed by one-way ANOVA followed by Tukey's multiple comparisons test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Expression analysis in haVSMC of genes associated to the acquisition of contractile and secretory phenotype. Relative expression levels of (A) ACTA2, (B) COL1A1, (C) TGFB1 and (D) MMP2 in haVSMC treated with vehicle, LTα1β2 (20ng/ml) and LTα1β2 (100ng/ml). mRNA levels were normalized with the levels of the endogenous gene and relativized to the vehicle-treated VSMC mRNA levels. Statistical analysis was performed by one-way ANOVA followed by Tukey's multiple comparisons test.
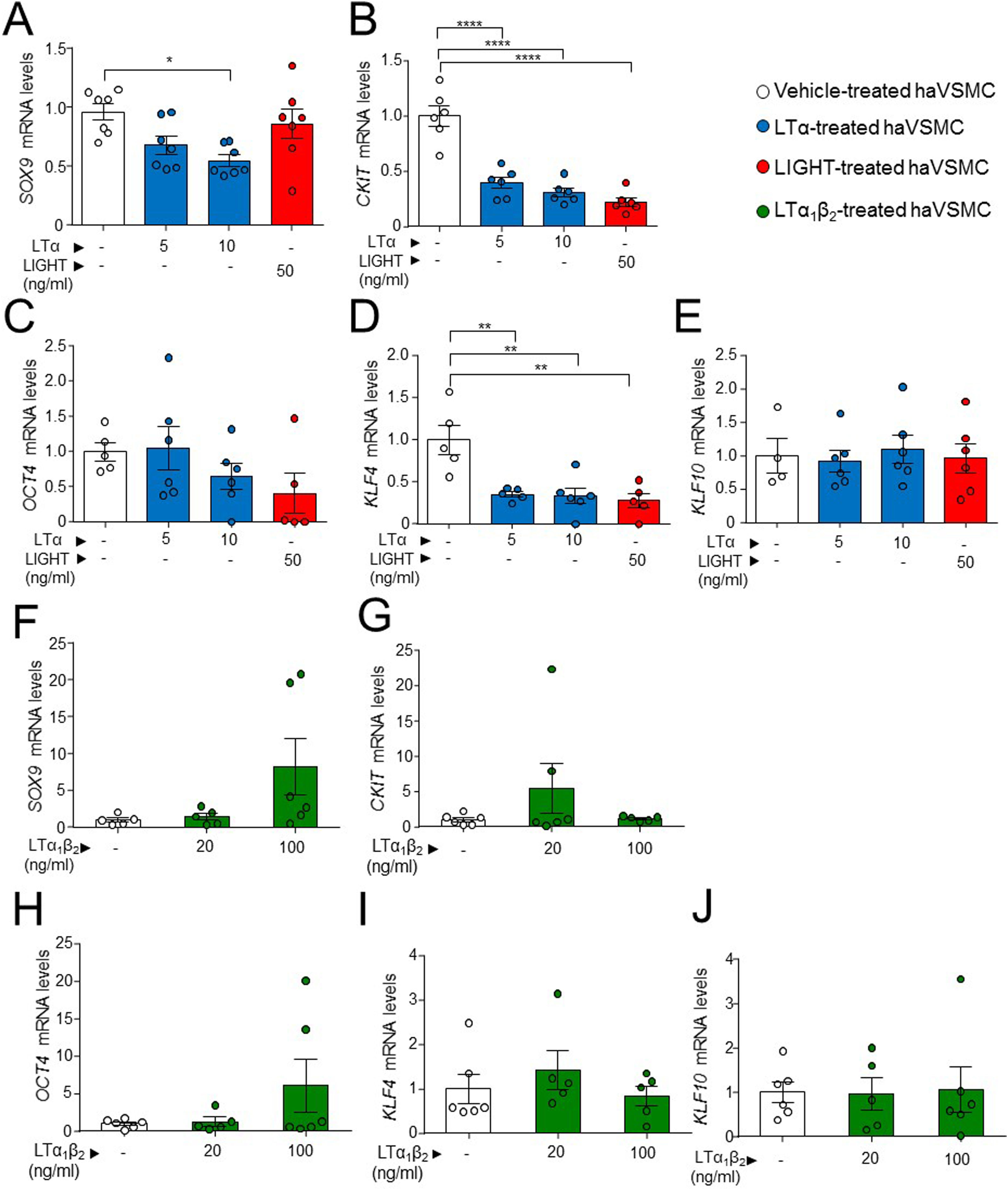
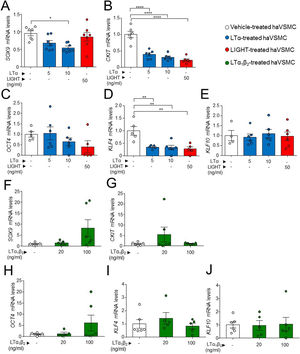
Next, we evaluated a possible effect of LIGHT and LT signaling in genes related with pluripotency and osteochondrogenic phenotypes. Treatment of haVSMCs with LTα at 10ng/ml, but not with LIGHT, significantly decreased the expression of SOX9 (Fig. 4A). Reduced mRNA levels of CKIT and KLF4 (Fig. 4B, C) were also observed in haVSMC treated with LIGHT and with LTα at the both 5 and 10ng/ml doses. Neither LIGHT or LTα affected the gene expression of OCT4 or KLF10 (Fig. 4D, E). Similarly, compared with vehicle-treated controls, haVSMCs treated with LTα1β2 did not display altered mRNA levels of SOX9, CKIT, OCT4, KLF4 or KLF10 (Fig. 4F–J).
Expression analysis of genes of pluripotency and osteochondrogenesis in haVSMC. Relative expression mRNA levels of (A, F) SOX9, (B, G) CKIT, (C, H OCT4, (D, I) KLF4 and (E, J) KLF10 in haVSMC treated with vehicle, LTα (5ng/ml), LTα (10ng/ml) and LIGHT (50ng/ml) (A–) and with vehicle, LTα1β2 (20ng/ml) and LTα1β2 (100ng/ml) (F–J). mRNA levels were normalized with the levels of the endogenous gene and relativized to the vehicle-treated VSMC mRNA levels. Statistical analysis was performed by one-way ANOVA followed by Tukey's multiple comparisons test. *p<0.05; **p<0.01;****p<0.0001.
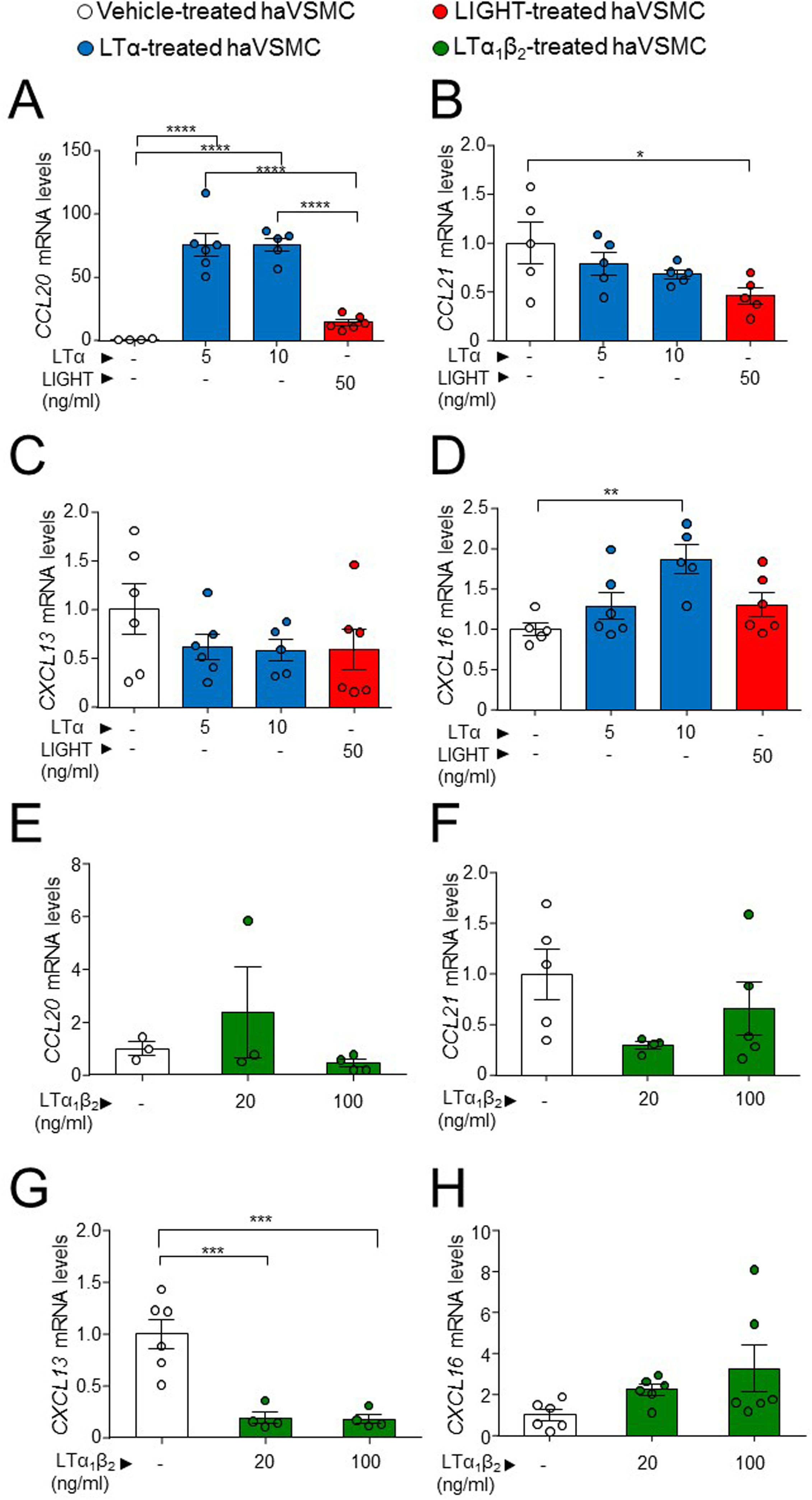
Aortic human vascular smooth muscle cells treated with LTα, LIGHT and LTα1β2 modulate the expression of lymphorganogenic cytokines
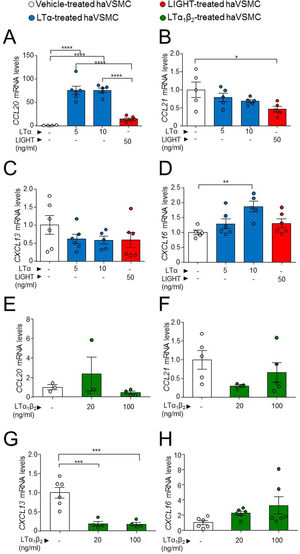
One of the described effects of LTβR-dependent signaling in VSMCs is the induction of a cellular phenotype secretor of lymphorganogenic cytokines, hence the production of these were studied. The analysis of haVSMC treated with LTα revealed an increase in the gene expression of CCL20 and CXCL16 at the 10ng/ml dose (Fig. 5A, D) and of CCL20 at 5ng/ml dose (Fig. 5A) compared with vehicle-treated VSMCs while the mRNA levels of CCL21 were unaffected by LTα (Fig. 5C). Unlike LTα, LIGHT did not modify the gene expression of CCL20 and CXCL16 (Fig. 5A, D) and surprisingly diminished CCL21 mRNA levels (Fig. 5B). The mRNA levels of CXCL13 were unaffected by either LTα or LIGHT treatment (Fig. 5C). Interestingly, the treatment of haVSMCs with the trimer LTα1β2, at both 20 and 100ng/ml doses, decreased the expression of the CXCL13 gene (Fig. 5G) while no changes were observed in the other cytokines (Fig. 5E, F, H).
Gene expression analysis of lymphorganogenic cytokines in haVSMC. Relative expression levels of (A, E) CCL20, (B, F) CCL21, (C, G) CXCL13 and (D, H) CXCL16 in ahVSMC treated with vehicle, LTα (5ng/ml), LTα (10ng/ml) and LIGHT (50ng/ml) (A–D) and with vehicle, LTα1β2 (20ng/ml) and LTα1β2 (100ng/ml) (E–H). mRNA levels were normalized with the endogenous gene levels and relativized to the vehicle-treated VSMC mRNA levels. Statistical analysis was performed by one-way ANOVA followed by Tukey's multiple comparisons test. *p<0.05; **p<0.01; ***p<0.001;****p<0.0001.
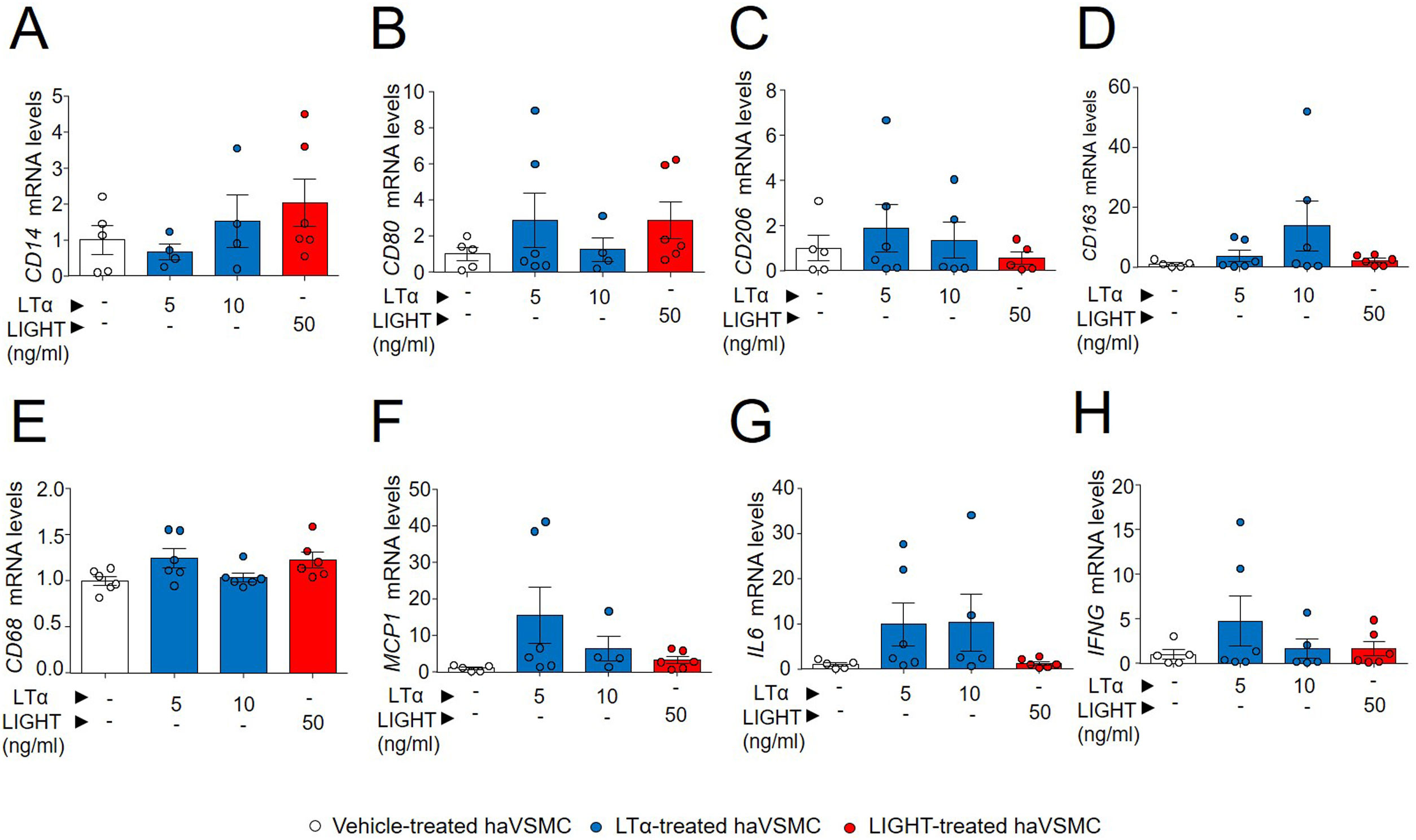
Previous studies have shown a modulation of macrophage-like cell phenotype through upregulation of KLF4.23 Given the lower KLF4 mRNA levels observed in haVSMCs treated with LIGHT and with LTα, the expression of macrophage gene markers were also investigated. Although the treatments with both LIGHT and LTα slightly increased the gene expression of the surface membrane markers of macrophage subtypes including CD14, CD80, CD206 and CD183 (Fig. 6A–D) and of phagocytic activity, CD68 (Fig. 6E) these were no significantly altered. Likewise, no differences were observed in the expression of proinflammatory cytokine genes typically expressed by macrophages, such as MCP1, IL6 and IFNG, between vehicle- and LTα- or LIGHT-treated haVSMCs (Fig. 6F–H).
Expression analysis of genes related to macrophage-like cell haVSMC. Relative expression levels of (A) CD14, (B) CD80, (C) CD206, (D) CD163, (E) CD68, (F) MCP1, (G) IL6 and (H) IFNG in haVSMC treated with vehicle, LTα (5ng/ml), LTα (10ng/ml) and LIGHT (50ng/ml). mRNA levels were normalized with the endogenous gene levels and relativized to the vehicle-treated VSMC mRNA levels. Statistical analysis was performed by one-way ANOVA followed by Tukey's multiple comparisons test.
Atheroma plaque instability is strongly affected by VSMC functional transdifferentiation5,22 in which different risk factors and secreted plaque mediators have been importantly implicated.24 In the present investigation, the potential effect of TNFSF ligands in the modulation of VSMC gene expression associated with functional heterogeneity was explored. Consistent with a preservation of VSMC identity, the treatment of VSMCs with LTα or LIGHT diminished COL1A1 and TGFB1 mRNA levels, LTα incubation augmented the expression of MMP9, without changing ACTA2 mRNA levels. Likewise, genes associated with osteochondrogenesis, pluripotency and transdifferentiation such as SOX9, CKIT, and KLF4 were also repressed by LTα and LIGHT treatments. Notably, all the above genes were not affected by the treatment with the LTα1β2 trimer. VSMC treatment with all three ligands, LTα, LTα1β2 and LIGHT, altered the expression of well-reported lymphorganogenic cytokines6,25 which included augmented CCL20 and CCL21 gene expression by LTα as well as diminished CCL21 and CXCL13 by LIGHT and LTα1β2, respectively. Altogether, indicates a role of the TNFSF ligands through their interconnected network of signaling, in the preservation of VSMC identity against the acquisition of a genetic expression signature compatible with a functional transdifferentiation process.
Studies have shown an important role of the cross-talk between the plaque-stress factors and the functional plasticity of VSMC. Thus, VSMC functionality loss consisting in an acquisition of an inflammatory and apoptotic phenotype promotes plaque instability in insulin resistance states through a CX3CL1-dependet manner.22 More recent investigations using cellular lineage tracing and single-cell RNAseq in plaque have further extended our knowledge about VSMC plasticity during plaque progression.23,26 Specifically, in vivo VSMC specific deficiency in Oct4 or Klf4 in mouse models resulted in two divergent genomic signatures associated with different VSMC fates and function such as osteochondrocyte-like and macrophage-like gene signatures.23,27 Notably, protective ACTA2+ myofibroblast-like cells forming the fibrous caps were discovered to originate from endothelial-to-mesenchymal transition and macrophage-to-mesenchymal transition, a process dependent of Pdgfrb gene.27
Our present investigation indicates a gene repression by LTα and LIGHT of COL1A1 and TGFB1, SOX9, CKIT, and KLF4 genes, results that are consistent with the above studies indicating important roles of some of these genes in plaque instability through VSMC phenotype switching. On the other hand, LIGHT/LTβR-dependent signaling has as well been linked to atherosclerosis with discrepant conclusions12,13,15–19 which we believe might be attributed to the intrincated network signaling of the TFNSF ligands. The present investigation is also in agreement with a study describing a protective function of LIGHT in aneurysm lesion20 and a prevention by LIGHT of SOX9 gene expression which is associated with an osteochondrogenic and proatherogenic phenotype. Consequently, in vivo analysis showed diminished ACTA2+ lesion area and vascular Col1a1 gene expression,20 suggesting a protective role for LIGHT by preventing VSMC de-differentiation and plasticity in vivo during vascular injury. Of note, as in the present investigation, LIGHT did not affect the in vitro gene expression of the ACTA2 contractile marker in haVSMCs which could be due to a high prevalence of ACTA2+ myofibroblast-like cells26 which could difficult the detection of other non-contractile cells originated during the transdifferentiation process. Hence, changes in gene expression were performed in the cell culture probably containing a mixed pool of cells with different cell-type phenotypes which could mask gene expression changes belonging to a less prevalent cell-type.
Therefore, a limitation of our results is that our study is based on gene expression techniques and hence future studies would require the detection of active proteins as well as the performance of functional assays to better characterize each cell-type. To avoid the above limitations, future studies should include experimental approaches such as single-cell RNAseq or flow-cytometry analysis using several markers to identify, fractionate and quantify the different cell-types induced by LTα, LTα1β2 and LIGHT.
In addition, a modulation by the TNFSF ligands of lymphorganogenic cytokines, including enhanced CCL20 and CCL21 gene expression and diminished CCL21 and CXCL13, has been also observed in the present investigation which suggest a coordinated role of these ligands in cytokine-mediated lymphorganogenesis. In the line of these results, VSMC Ltbr-specific deficiency in mice prevented this cell type to acquire a protective lymphorganogenic PDGFRB+ phenotype,19 and aggravated lesion size, suggesting a role in lymphorganogenesis associated with plaque stability.
Altogether, the present investigation indicates a role of the TNFSF ligands in VSMC gene expression of genes relevant for cellular plasticity and reprogramming events during plaque lesion development and stability. Therefore, these data suggest a possible role of these ligands in cellular plasticity during atheroma development.
ConclusionsThe TNFSF ligands through its interconnected network signaling modulate haVSMC gene expression signature associated with cellular plasticity. These results suggest a possible role of the TNFSF ligands in plaque stability.
FundingThis research was funded by grants from the ISCIII (PI19/00169 to H.G.-N. and S.M.-H. and CIBERDEM CB07/08/0043, CB07/08/0018), the European Regional Development Fund (FEDER), GenT Investigator (CDEI-04-20-B, GVA) and by the Spanish Arteriosclerosis Society (BIB 07-20). MAB received support from a fellowship of ISCIII and the European Regional Development Fund (FEDER) (FI20/00017) and GHG from the Ministerio de Universidades (FPU20/04916).
Conflict of interestsThe authors have no conflict of interests to declare.