To investigate the association of body cell mass loss with disease activity and disability in rheumatoid arthritis patients.
INTRODUCTION:Rheumatoid cachexia, defined as the loss of body cell mass, is important but under-recognized and contributes to morbidity and mortality in patients with rheumatoid arthritis.
METHODS:One hundred forty-nine rheumatoid arthritis patients and 53 healthy, non-rheumatoid arthritis control subjects underwent anthropometric measurements of body mass index and waist and hip circumferences. Bioelectrical impedance analysis was used to determine the subjects' body compositions, including fat mass, skeletal lean mass, and body cell mass. The disease activity of rheumatoid arthritis was assessed using C-reactive protein serum, the erythrocyte sedimentation rate and the 28-joint disease activity score, while disability was evaluated using a health assessment questionnaire.
RESULTS:Rheumatoid arthritis patients had lower waist-to-hip ratio (0.86±0.07 vs. 0.95±0.06; p<0.001) and lower skeletal lean mass indexes (14.44±1.52 vs. 15.18±1.35; p = 0.002) than those in the healthy control group. Compared with rheumatoid arthritis patients with higher body cell masses, those with body cell masses lower than median had higher erythrocyte sedimentation rates (40.10±27.33 vs. 25.09±14.85; p<0.001), higher disease activity scores (5.36±3.79 vs. 4.23±1.21; p = 0.022) and greater disability as measured by health assessment questionnaire scores (1.26±0.79 vs. 0.87±0.79; p = 0.004).
CONCLUSIONS:The loss of body cell mass is associated with higher disease activity and greater disability in rheumatoid arthritis patients. Body composition determined by bioelectrical impedance analysis can provide valuable information for a rheumatologist to more rapidly recognize rheumatoid cachexia in rheumatoid arthritis patients.
Rheumatoid cachexia affects two-thirds of rheumatoid arthritis (RA) patients and is defined as the loss of body cell mass (BCM), which is the fat-free component of cells within muscle, visceral organs and the immune system, and an often compensatory increase in fat mass (FM; i.e., cachectic obesity).1 BCM is considered to be the most important factor in determining energy expenditure, protein needs, and the metabolic response to stress.2 The consequences of chronic inflammation and increased production of cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-1β, are responsible for higher resting energy turnovers and altered body compositions in RA patients.3 Sir James Paget described wasting of skeletal muscle mass in patients with inflammatory arthritis that was not due to disuse atrophy.4 The loss of BCM in various diseases, including RA, congestive heart failure, acquired immunodeficiency syndrome (AIDS), starvation, critical illness, and aging has been associated with poor clinical outcomes.5 Rheumatoid cachexia is associated with an increased erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), functional dependence, and an increased chance of morbidity and premature mortality.6 Although the prevalence rate of rheumatoid cachexia is high, it remains under-recognized, partly because an abnormal body composition phenotype in RA patients occurs most often in patients with normal body mass indexes (BMIs).1,3,7–9 Therefore, a body composition measurement beyond anthropometric parameters is essential to identifying RA patients with rheumatoid cachexia.
A wide range of imaging techniques have been used to analyze the body compositions of RA patients, and dual X-ray absorptometry (DXA) has been reported to be the most useful tool for measuring soft tissue mass and bone mineral density.3,6,10–12 However, DXA is not universally available, requires a scheduled appointment and is sensitive to the patient's hydration status.13–15 A rapid and simple tool for identifying rheumatoid cachexia in outpatient settings is therefore necessary.
The abilities of different body tissues to conduct electrical currents have been known for more than a century.16 Bioelectrical impedance analysis (BIA) has the ability to distinguish fat tissue from fat-free tissue and water. Due to its relatively low cost, easy operation and high portability, BIA is probably the most commonly utilized method of evaluating body composition.16
The present study was designed to compare the body compositions, as measured by BIA, of RA patients with those of healthy control subjects. The impact of RA disease activity and disability on body composition was also explored.
MATERIALS AND METHODSParticipantsThis study included 149 patients who visited the rheumatology clinic of Taichung Veterans General Hospital and were diagnosed with RA according to the 1987 revised criteria of the American College of Rheumatology (ACR).17 Patients with terminal cancer, end-stage liver, or renal disease were excluded. Fifty-three age- and gender-matched volunteers without rheumatic diseases were enrolled as healthy control subjects.
Anthropometric MeasurementsAll measurements were performed after a 12-hour overnight fast. Participants were weighed while wearing light clothes but no shoes. Patient heights were determined to the nearest 0.1 cm using a fixed-wall-scale measuring device. The weight of each subject was determined to an accuracy of 0.1 kg using an electronic scale that was calibrated before each measurement session. The BMI was calculated as weight (kg) per height (m2). The waist circumference (WC) was measured to the nearest centimeter at the level of the umbilicus after expiration while the participant was standing still, breathing quietly, distributing weight equally onto both feet with arms hanging loosely at the sides and the head facing straight ahead. The hip circumference (HC) was measured to the nearest centimeter at the level of the maximal protrusion of the gluteal muscles over the underwear. The waist-to-hip ratio (WHR) was computed as WC divided by HC.
Body composition determinationThe patients' body compositions, including the FM and the skeletal lean mass (SLM), were assessed by the InBody 720 device (Biospace Co., Korea), a multifrequency impedance meter that takes readings from the body using an eight-point tactile electrode method and measures resistance at five frequencies (1 kHz, 50 kHz, 250 kHz, 500 kHz, and 1 MHz) and reactance at three frequencies (5 kHz, 50 kHz, and 250 kHz).18–20 In accordance with the manufacturer's guidelines, participants wiped the bottom of their feet with a proprietary electrolyte tissue before standing on the electrodes embedded into the scale platform. The participants were instructed to stand upright and grasp the handles of the analyzer, thereby providing contact with a total of eight electrodes (two for each foot and hand). The data were electronically imported into Excel using the software Lookin'Body 3.0. The repeated FM measurements exhibited a coefficient of variation of 0.6%. The FM index was computed as the FM per height (m2), while the SLM index was calculated as the SLM per height (m2). BCM was determined according to sex-specific equations that have previously been validated and reported.21
RA disease activityThe CRP serum levels, ESR and the 28-joint disease activity score (DAS28)22 were utilized to assess the RA disease activity. Low disease activity was defined as DAS28 ≦ 3.2, moderate disease activity was defined as DAS28>3.2 but ≦ 5.1, and high disease activity was defined as DAS28>5.1.23
DisabilityThe level of disability in terms of disease impact was determined by a health assessment questionnaire (HAQ),24 which is a 20-item self-reported functional questionnaire with items rated from 0 to 3 and increasing in steps of 0.125 units. Subjects are considered to present a disability if their HAQ score≧1.25
EthicsThis study protocol was approved by the Ethics Committee of Clinical Research, Taichung Veterans General Hospital, and informed consent was obtained from each participant.
Statistical AnalysisThe data in the text and tables are expressed as a mean±standard deviation. The software Statistical Package for the Social Sciences (SPSS) version 13.0 (SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses. Student's t-test or the Mann-Whitney U test were used to compare continuous variables when appropriate. Categorical variables were compared using the chi-squared test or Fisher's exact test when appropriate. Associations between variables were determined by Pearson's correlation. For all tests, a p-value <0.05 was considered statistically significant.
RESULTSDemographic data, clinical characteristics, anthropometric measurements, body composition and comorbidities in RA patients and healthy control subjectsAs shown in Table 1, RA patients exhibit significantly lower waist-to-hip ratios than do healthy controls (0.86±0.07 vs. 0.95±0.06, respectively; p<0.001) and exhibit lower SLM indexes than do healthy controls (14.44±1.52 vs. 15.18±1.35, respectively; p = 0.002). The RA patients also exhibited a trend towards decreased BCM compared to the control group (24.11±3.64 kg vs. 25.12±3.62 kg, respectively; p = 0.083), although statistical significance was not achieved. No significant differences were observed in the demographic data or comorbidities of the RA patients and the healthy control subjects.
Demographic data, comorbidities and body compositions of 149 patients with rheumatoid arthritis (RA) and 53 unrelated healthy control subjects (HC)#.
| Factors | RA (n = 149) | HC (n = 53) |
|---|---|---|
| Age at study entry (years) | 56.68±12.52 | 60.11±8.94 |
| Gender (male, %) | 18 (12.1) | 7 (13.2) |
| BMI (kg/m2) | 24.08±4.23 | 25.20±3.46 |
| WC (cm) | 79.72±10.28 | 88.04±9.84** |
| HC (cm) | 92.94±8.55 | 92.38±7.10 |
| Waist-to-hip ratio | 0.86±0.07 | 0.95±0.06** |
| ESR (mm/hr) | 33.12±23.56 | NA |
| CRP (mg/dl) | 1.60±9.09 | NA |
| DAS28 | 4.84±2.96 | NA |
| HAQ | 1.08±0.81 | NA |
| FM (kg) | 21.37±8.26 | 21.63±6.46 |
| FM index (kg/m2) | 8.79±3.53 | 9.06±3.01 |
| SLM (kg) | 35.40±5.21 | 36.70±5.23 |
| SLM index (kg/m2) | 14.44±1.52 | 15.18±1.35** |
| BCM (kg) | 24.11±3.64 | 25.12±3.62 |
| DM | 6 (4.1) | 4 (7.5) |
| Hypertension | 54 (37.2) | 17 (32.1) |
| Smoking | 11 (7.6) | 2 (3.8) |
BMI: body mass index; WC: waist circumference; HC: hip circumference; WHR: waist-to-hip ratio; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; DAS28: 28-joint disease activity score; HAQ: health assessment questionnaire; FM: fat mass; SLM: skeletal lean mass; DM: diabetes mellitus.
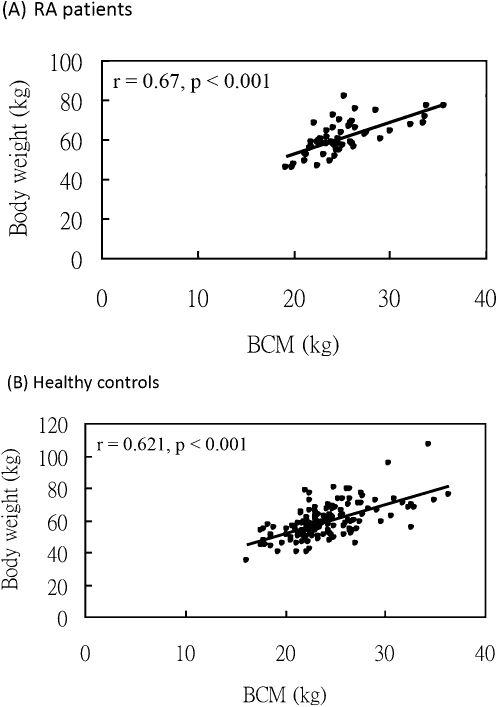
As shown in Figure 1, body weight and BCM are positively and significantly correlated in both RA patients (A, correlation coefficient = 0.62; p<0.001) and healthy control subjects (B, correlation coefficient = 0.67; p<0.001).
Comparison of clinical characteristics, anthropometric measurements and body compositions of RA patients with different BCM levelsThe RA group was split into two groups, with BCM values higher or lower than the gender-stratified median BCM values (23.4 in female and 29.2 in male participants, see Table 2). Patients with lower BCM values were older than were patients with higher BCM values (60.14±11.45 years vs. 52.70±12.59 years, respectively; p<0.001), had higher ESR levels (40.10±27.33 mm/hr vs. 25.09±14.85 mm/hr, respectively; p<0.001), had higher DAS28 values (5.36±3.79 vs. 4.23±1.21 respectively; p = = 0.022), were more physically dependent in terms of HAQ scores (1.26±0.79 vs. 0.87±0.79, respectively; p = 0.004), and exhibited decreased serum albumin levels (3.97±0.44 vs. 4.17±0.51, respectively; p = 0.041).
Comparison of clinical characteristics, anthropometric measurements and body compositions of RA patients with diffebody cell mass (BCM) levels#.
| BCM | BCM ≧ median (n = 70) | |
|---|---|---|
| Age (years) | 60.14 ±11.45 | 52.70±12.59** |
| Male (n, %) | 9 (11.4) | 9 (12.9) |
| BMI (kg/m2) | 21.86±2.36 | 26.65±3.13** |
| WHR | 0.87±0.08 | 0.89±0.07 |
| ESR (mm/hr) | 40.10±27.33 | 25.09±14.85** |
| CRP (mg/dl) | 0.75±1.39 | 0.44±0.81 |
| DAS28 | 5.36±3.79 | 4.23±1.21* |
| HAQ | 1.26±0.79 | 0.87±0.79* |
| Albumin (mg/dl) | 3.97±0.44 | 4.17±0.51* |
| BCM (kg) | 23.11±3.75 | 25.18±4.50* |
| FM (kg) | 19.77±7.05 | 23.17±9.15* |
| FM index | 8.44±3.21 | 9.19±3.85 |
| SLM | 32.22±3.39 | 38.98±4.53** |
| SLM index | 13.66±1.10 | 15.32±1.45** |
| Prednisolone (mg/day) | 6.09±2.39 | 5.64±3.05 |
| TNF-α inhibitors (n, %) | 54 (68.4) | 41 (62.1) |
| DM | 2 (2.5) | 4 (5.7) |
| Hypertension | 33 (41.7) | 21 (30.0) |
| Smoking | 5 (6.3) | 6 (8.6) |
RA: rheumatoid arthritis; BCM: body cell mass; BMI: body mass index; WHR: waist-to-hip ratio; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; DAS28: 28-joint disease activity score; HAQ: health assessment questionnaire; FM: fat mass; SLM: skeletal lean mass; TNF-α inhibitors: tumor necrosis factor-α inhibitors;
DM: diabetes mellitus.
The median BCM values were 23.4 and 29.2 in females and males, respectively.
In Figure 2A, RA patients with high disease activity had lower BCMs than did those with moderate or low disease activity. Moreover, RA patients with physical dependence in terms of HAQ scores had lower BCMs than did those without a disability (Figure 2B).
(A) Comparisons of body cell mass (BCM) values of RA patients with low, moderate and high levels of disease activity by analysis of variance. (B) Comparisons of BCM values of RA patients with and without disability by independent sample t-test. Values are presented as box-plot diagrams, with the box encompassing the range from the 25th percentile (lower bar) to the 75th percentile (upper bar). The horizontal line within the box indicates the median value, and the horizontal lines above and below the box represent the maximum and minimum values, respectively, for each group.
Rheumatoid cachexia, characterized by the loss of BCM, leads to an increased chance of morbidity and premature mortality.6 BCM has also been reported as a valuable indicator of nutritional status, physical activity, and RA disease activity, making it a powerful predictor of outcome in various disease entities, including RA, critical illness, starvation, and aging.26,27 Anthropometric measurements including body weight and BMI, although useful in predicting disease activity and survival in RA patients, are not sensitive to the changes in body composition that occur with aging and chronic inflammation.6 Therefore, it is important to analyze body composition using tools beyond simply measuring body weight and BMI to evaluate the impact of chronic inflammation in each individual.28 The present study is the first attempt to measure FM, SLM, and BCM, which are surrogate markers of rheumatoid cachexia, in RA patients of Asian ethnicity using BIA technology, which is quick and easy to deploy in the outpatient setting of rheumatology clinics.
Investigations of differences in the body compositions of RA patients and healthy control subjects are inconclusive in the literature.6 Our results and those of previous studies found no difference in the BMI values of RA patients and healthy control subjects.3,7 The loss of lean body mass or BCM in RA patients has previously been measured using a variety of techniques, including anthropometric measurements, DXA, and potassium-40.3,7,29 The increased prevalence of skeletal mass loss and fat mass gain in RA patients is related to joint deformity, physical inactivity, and poor clinical outcomes.9 Therefore, it is crucial to recognize abnormal body composition phenotypes as early as possible. By using BIA, a simple and reliable body composition measurement, our study observed reduced SLM indexes and BCM values in RA patients when compared with healthy control subjects.
Whether FM is increased or not in RA patients has been controversial in the past. A non-significant increase in fat mass has been reported in previous studies,29 while Roubenoff et al reported a reduction in FM.7 However, Stavropoulos-Kalinoglou et al found that at a given BMI, RA patients exhibited higher proportions of body FM than healthy control subjects.30 In the present study, the FMs of the RA group and the healthy control group were indistinguishable. The diverse results regarding fat mass in RA patients may be due to the different ethnicities, patient characteristics, and methods of measurement used in different studies.
The central pathogenesis of RA involves chronic inflammation due to overproduction of the inflammatory cytokines TNF-α and IL-1β, which are also critical to whole body protein degradation and muscle wasting.1 Significant correlations have been reported between rheumatoid cachexia and disease activity, including the number of swollen joints, CRP and ESR.7 Our results demonstrate a significant association among the loss of BCM observed through BIA, the ESR and the DAS28, one of the most commonly utilized scoring systems for evaluating RA disease activity.22
In RA, functional limitations to daily activity originate mainly from joint pain, swelling, and deformity.31 However, decreased SLM may contribute to a substantial loss of muscle strength and endurance and result in decreased manual dexterity, difficulty in locomotion, reduced work productivity, and impaired quality of life.32 A recent study exploring the association between body composition and disability in RA provided the valuable finding that loss of SLM in the arms and legs is strongly linked to functional limitation.33 In the present study, we found that in Chinese RA patients, loss of BCM is significantly associated with disability in terms of HAQ scores.
Cigarette smoking has been demonstrated to increase the rest energy expenditure of RA patients, which could potentiate rheumatoid cachexia in these patients.34 Moreover, smoking has been reported to be associated with reduced BMI and body fat, while heavy smoking has been linked with lower muscle mass in RA patients.35 In our study, the fraction of smokers among the RA patients appeared to be higher than that of the healthy control subjects (7.6% vs. 3.8%, respectively), although statistical significance was not achieved. The higher proportion of smokers could partly explain the decreased SLM indexes of RA patients. However, there was no difference in the number of participants with higher and lower BCM values.
This study does have some limitations. First, the study design was cross-sectional, which may limit the applicability of the results. However, the study results demonstrated close associations between BIA-derived body composition parameters and several clinical variables in RA patients. Second, body compositions were assessed by BIA, a method that has not been specifically validated in the RA population. Nevertheless, BIA provides a rapid and simple way to identify patients at risk of rheumatoid cachexia in outpatient settings. Third, this study was an observational investigation, and the effects of appropriate intervention for rheumatoid cachexia remain to be elucidated. Therefore, a prospective, controlled study with intervention programs is needed.
CONCLUSIONSLoss of BCM is associated with disease activity and disability in Chinese RA patients. BIA may be a quick, convenient and inexpensive tool to assess body composition and facilitate early recognition of rheumatoid cachexia by rheumatologists during everyday clinical practice.
The authors are grateful to the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung Taiwan, ROC for statistical analysis for this study.