To investigate the impact of transgastric peritoneal access on plasma biomarkers of acute inflammatory response in comparison to laparoscopy.
METHODS:This was a prospective and comparative study in a porcine model. Transgastric peritoneal access performed by natural orifice transluminal endoscopic surgery was compared with laparoscopy. Laparotomy and sham groups were used as positive and negative controls, respectively. Thirty-four pigs were assigned to receive transgastric natural orifice transluminal endoscopic surgery (n = 12), laparoscopy (n = 8), laparotomy (n = 8) or a sham procedure involving only anesthesia (n = 6). In the natural orifice transluminal endoscopic surgery group, peritoneoscopy was performed with a gastroscope via transgastric access. Blood samples were collected at baseline and 1, 3, 6, 9 and 24 h after the surgical procedure for measurement of interleukins 1β, 6 and 10 and tumor necrosis factor-α. A complete blood count was performed, and C-reactive protein levels were measured at baseline and at 24 h.
RESULTS:All surgical and endoscopic procedures were performed without major complications. Peritoneal cavity inventory showed no signs of peritonitis in any animal. Interleukin 1β, interleukin 10 and tumor necrosis factor-α levels were below the threshold of detection. The mean level of interleukin 6 was statistically significantly higher in the laparotomy group than in the other groups (p<0.05), with no significant differences among the sham, laparoscopy and natural orifice transluminal endoscopic surgery groups (p>0.05). C-reactive protein analysis indicated significant increases in all groups, with no differences among the groups. Complete blood count analysis showed no differences among the groups.
CONCLUSIONS:Based on the observed interleukin 6 patterns, the systemic inflammatory response resulting from transgastric peritoneal access by natural orifice transluminal endoscopic surgery is similar in intensity to the response that occurs after laparoscopy.
Natural orifice transluminal endoscopic surgery (NOTES) has been the object of organized scientific effort as a potentially minimally invasive means of peritoneal access (1,2). Although it is natural to imagine that NOTES should result in less intense local and systemic inflammatory responses than conventional surgical approaches because no external incisions are performed, many questions remain unanswered concerning the real benefits that this new procedure could bring to patients (3). With NOTES, access to the peritoneal cavity is achieved by inserting a non-sterile endoscope through a hollow contaminated viscus such as the stomach or colon or through the vagina, with potential complications such as fistulas and infection (4). Research on inflammatory responses to surgery has frequently used cytokines (e.g., interleukin [IL]-6, IL-1β, IL-10 and TNF-α) and acute phase proteins (e.g., C-reactive protein [CRP]) as plasma biomarkers of the inflammatory response (5,6). It is already known that laparoscopic surgery has less impact on the inflammatory response than laparotomy (7-13). However, to date, few reports have compared NOTES to laparoscopy with respect to the systemic impact of using transgastric access to the peritoneum, and the few studies that have addressed this issue report conflicting results (14-19).
The purpose of this study was to evaluate the acute inflammatory systemic response to NOTES by analyzing plasma biomarkers of the acute inflammatory response 24 hours after transgastric NOTES peritoneoscopy in a porcine model.
MATERIALS AND METHODSThis report describes a prospective, comparative and non-randomized study in a porcine model. To compare NOTES with laparoscopy, an experimental study was designed assuming laparotomy as the positive control (higher impact on the inflammatory response) and a sham group, which received only anesthesia, as the negative control. Animals were assigned to receive transgastric peritoneoscopy (NOTES, n = 12), laparoscopy (n = 8), laparotomy (n = 8) or a sham procedure (SHAM, n = 6).
All procedures were performed by one endoscopist with training in NOTES (NOTES group) and a surgeon with experience in laparoscopy (laparoscopy and laparotomy groups). This non-survival study was conducted at the Federal University of São Paulo, Brazil, after approval by our Research Ethics Committee. A total of 42 Sus scrofa domesticus (C-76 Agroceres®, São Paulo, Brazil) male pigs, aged approximately 12 weeks and weighing between 30 and 40 kg, were used in this study.
The preoperative care protocol was identical for all groups. Animals were fed appropriate standard swine feed and were given water ad libitum until they were transferred to individual stalls with wooden pallets, where they were kept for a 2-day period for acclimation before surgical procedures. During this acclimatization period, they were fasted for 12 hours before the procedure. Animals were evaluated by a veterinarian to assure their baseline health and their suitability for the study; the evaluation included weight, confirmation of male sex, rectal temperature and complete blood count (CBC), ensuring a homogeneous group of animals for study.
Anesthesia, surgical procedures and postoperative careProtocols for sedation, anesthesia, ventilation and postoperative care were identical for all groups. With the assistance of a veterinary anesthesiologist, animals were sedated with ketamine (5-7.5 mg/kg) and midazolam (0.25-0.35 mg/kg) IM before initiation of the procedures. The lateral auricular vein at the back of the ear was punctured, and pre-oxygenation was provided with a catheter adapted to the nasal fossa. The animals received propofol (5 mg/kg) followed by endotracheal intubation. Anesthesia was maintained with 2.5% isoflurane under controlled ventilation for a minimum of 90 minutes in all study groups. Blood pressure, temperature, pulse and oxygen saturation were monitored during the procedure. No animals received venous antibiotics before or after the procedure. All surgical procedures, including jugular vein dissection, laparoscopy and laparotomy, were performed under sterile conditions. For the NOTES group, the accessories used were sterile, while the endoscope was processed with high-level disinfection.
To collect blood samples over the 24-hour period following the procedure, the right internal jugular vein was dissected, and access was maintained with a catheter with heparin solution. As soon as the catheter was in place, the first blood samples were collected (T0 = 0 min); the procedure was then performed according to the animal's assigned group. At the end of the procedure, the animals were returned to their individual stalls, where they remained until the end of the experiment (T24 = 24 h after T0). Standard analgesia (IM tramadol 100 mg) was used at the end of the procedure and again 8 h later. The animals were permitted to drink water during the recovery time following the procedure. For 24 h following the procedure, a veterinarian monitored the animals and classified them according to three recovery levels at T3, T9 and T24: sedated (score 1) - when the animal was lying down with eyes closed; hypoactive (score 2) - when the animal was lying with minimal movements and eyes open; and active (score 3) - when the animal was walking with eyes open.
Sham group (negative control with anesthesia only)Animals received anesthesia for 90 minutes with no other procedure except for jugular vein dissection.
Transgastric NOTES peritoneoscopy (NOTES group)Animals received anesthesia and underwent transgastric peritoneal access and peritoneoscopy. After oral cavity decontamination with chlorhexidine, upper digestive endoscopy was performed with saline irrigation followed by aspiration of any residue remaining in the stomach. A second irrigation was performed with cephalothin (1 g) diluted in 1000 ml of saline, followed by complete stomach aspiration. Endoscope passage was facilitated using an overtube placed down to the esophageal-gastric junction.
To gain access to the peritoneum, a submucosal tunnel was carried out using the procedure described by Yoshizumi et al. (20) with a minor modification using a hot-biopsy forceps for submucosal dissection. Inventory of the peritoneal cavity was performed using a gastroscope (EG-250WR5, Fujinon Corp., Japan) through the gastric puncture. Pneumoperitoneum was obtained by insufflating room air provided by the gastroscope, without controlling pressure but avoiding excessive abdominal distension and respiratory distress. During peritoneoscopy, the main abdominal organs were recognized, and a regular biopsy forceps was used to assist the manipulation and visualization of visceral structures. Once the inventory had been completed, the air was aspirated using the endoscope, which was then removed. The endoluminal proximal incision of the tunnel was closed with metallic clips (EZ Clip, Olympus Optical) or with 0.5 ml of diluted cyanoacrylate (Histoacryl®, B. Braun, Germany).
Laparoscopy groupAnimals received anesthesia and underwent video laparoscopic access and peritoneoscopy. The abdomen was prepared in a sterile manner. A Veress needle was introduced into the abdomen's lower left quadrant, and pneumoperitoneum was obtained by insufflating carbon dioxide until a pressure of 12 mm Hg was reached. Two additional trocars were then placed followed by an optic device and dissection forceps to assist in the peritoneoscopy. The peritoneal cavity was examined, targeting the same structures described for the NOTES group. When peritoneoscopy had been completed, the trocar was opened, and the gas was released. The trocars were removed, and the abdominal wall was sutured.
Laparotomy groupAnimals received anesthesia, laparotomy and peritoneal cavity inventory. The abdomen was prepared in a sterile manner, and a long xifopubic skin incision was created. After dissection of the abdominal wall layers, inventory of the peritoneal cavity was performed, with observation of the same structures as for the NOTES and laparoscopy groups. The abdominal wall layers were then sutured.
Data collection and cytokine measurementsBlood samples were obtained during the experiment at baseline (T0 = 0 min, after jugular vein dissection and immediately before beginning the main procedure) and at 1, 3, 6, 9 and 24 h after T0 (T1, T3, T6, T9 and T24, respectively) for measurement of the cytokines IL-1β, IL-6, IL-10 and TNF-α. Additional samples were collected at T0 and T24 for CBC and CRP determination.
For cytokine and CRP analysis, blood was collected in three heparinized tubes and centrifuged to obtain plasma aliquots, which were frozen and stored at -80°C until analysis. Immunoassays for IL-6, IL-1β, IL-10, TNF-α and CRP were performed using commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturers' instructions: IL-6, IL-1 and IL-10 (R&D Systems, Inc., Minneapolis, MN, USA); TNF-α (Swine TNF-α CytoSetTM, Invitrogen, BioSource International, Inc., Camarillo, CA, USA); and CRP (Pig CRP ELISA Kit®, Immunology Consultants Laboratory, Inc. Newberg, OR, USA). The results are expressed in pg/ml. Blood samples for CBC were analyzed according to standard techniques.
Euthanasia and inventory of the peritoneal cavityTwenty-four hours after the procedure, the animals were sedated with propofol and euthanized by intravenous injection of 19.1% potassium chloride. Peritoneal cavity inventory was performed in all animals to detect any signs of peritonitis (presence of serous exudate, pus, abscess or adherences), the presence of blood or any accidental injury to the abdominal organs. For animals in the NOTES group, the anatomic block including the stomach with the distal esophagus and the proximal duodenum was resected to check tunnel closure and to perform a leakage test to ensure gastrostomy closure. The leakage test was made with the ex vivo stomach immersed in water and inflated with air for 1 minute. The test was considered negative if no bubble was observed. After the leakage test, the stomach was opened and examined to verify the tunnel characteristics.
Statistical analysisNormal distribution for each parameter was ascertained using the Kolmogorov-Smirnov test; parameter values are given as means with standard deviations. Comparisons among groups regarding weight, temperature, surgical time and anesthesia time were conducted using one-way analysis of variance (ANOVA), and the Kruskal-Wallis test was used to compare postoperative recovery among groups.
Cytokine values were first analyzed including values from all animals. However, to eliminate baseline variability in the cytokine levels obtained before the procedure and to analyze the within-group and among-group differences in cytokine levels, the data are expressed as the difference between the value obtained at each sampling time and the baseline value. This difference was calculated as a percentage in the following manner: (postoperative value minus preoperative value at baseline divided by preoperative value at baseline) x 100. For IL-6, the differences (%) among and within groups (along time) were evaluated using a linear model with a first-order autoregressive correlation structure applied to the difference (%) for each time assessed. When the test's sensibility was equal to zero, the plate's threshold value was attributed. For CRP measurements and CBC variables, differences were assessed using repeated measures analysis of variance (ANOVA). To assess differences between groups in the presence of interaction, Bonferroni's correction method for multiple comparisons was used.
A p<0.05 was considered statistically significant. Statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTSEight of 42 animals were excluded from the study because of complications or protocol violations that could potentially interfere with the systemic inflammatory response. For the remaining 34 animals, the experimental protocols were concluded and analyzed. No statistically significant differences were identified among animals with respect to weight, preoperative rectal temperature or anesthesia mean times (p>0.05, Table 1). A significant difference was observed among all groups with respect to surgical mean times (p<0.05, Table 1).
Weight, rectal temperature, surgical time and anesthesia time of the animals used in this study.
| Sham | NOTES | Laparoscopy | Laparotomy | p-value* | |
|---|---|---|---|---|---|
| n = 6 | n = 12 | n = 8 | n = 8 | ||
| Weight (kg) | 32.6 (1.1) | 33.7 (0.8) | 34.6 (1.2) | 32.7 (1.1) | NS |
| Temperature (°C) | 37.6 (0.2) | 37.4 (0.1) | 37.6 (0.3) | 37.4 (0.2) | NS |
| Surgical time (min) | - | 57.4 (4.5) | 20.8 (3.7) | 39.9 (3.6) | <0.001 |
| Anesthesia time (min) | 96.7 (5.7) | 97.2 (8.4) | 88.1 (1.9) | 93.2 (3.1) | NS |
Data are expressed as means (standard deviations). *: One-way ANOVA; Bonferroni-adjusted test indicated a significant difference among all of the groups; NS: not significant.
The animals in the laparoscopy, NOTES and sham groups did not present significant differences in postoperative recovery scores, while animals in the laparotomy group presented lower scores (p<0.05, Table 2).
Postoperative recovery score.
| Postoperative | Sham | NOTES | Laparoscopy | Laparotomy | p-value* |
|---|---|---|---|---|---|
| n = 6 | n = 12 | n = 8 | n = 8 | ||
| T3 | 3 (2-3) | 2 (1-3) | 3 (2-3) | 2 (2-2) | <0.05 |
| T9 | 3 (2-3) | 3 (2-3) | 3 (3-3) | 2 (2-3) | <0.05 |
| T24 | 3 (2-3) | 3 (2-3) | 3 (3-3) | 2 (2-3) | <0.05 |
T3, T9 and T24: 3, 9 and 24 h after procedure. Data are expressed as medians (minimum and maximum values). *: Kruskal-Wallis test.
All surgical and endoscopic procedures, including peritoneoscopy, were performed with no major complications. Peritoneal cavity inventory showed no signs of peritonitis in any animal. The leakage test was negative in all NOTES animals. The mean length of the tunnel was 4.6 cm (range from 2 to 6.5 cm).
The complete blood count (CBC) data are presented in Table 3. All groups exhibited significant increases in hematocrit and hemoglobin values after 24 hours (p<0.05), with no significant differences among groups. There was no difference in total leucocyte or segmented leucocyte values at T24. Both the sham and laparotomy groups presented a significant increase in platelet count at T24 (p<0.05), while the NOTES and laparoscopy groups did not present significant differences.
Complete blood count measurements at T0 and T24.
| Time (hours) | Sham | NOTES | Laparoscopy | Laparotomy | p-value* (Group) | |
|---|---|---|---|---|---|---|
| n = 6 | n = 12 | n = 8 | n = 8 | |||
| Hematocrit (%) | 0 | 31.9 (0.8) | 30.9 (1.0) | 34.9 (1.1) | 31.9 (0.8) | NS |
| 24 | 33.7 (0.9) | 31.5 (1.4) | 35.1 (0.9) | 33.5 (0.9) | ||
| Hemoglobin (g/dl) | 0 | 10.6 (0.3) | 9.9 (0.4) | 11.2 (0.3) | 10.7 (0.2) | NS |
| 24 | 11.2 (0.3) | 10.2 (0.5) | 11.2 (0.3) | 11.2 (0.3) | ||
| Total leucocytes (x 1000/μl) | 0 | 13.0 (1.3) | 14.4 (1.0) | 14.2 (1.2) | 14.0 (1.6) | NS |
| 24 | 13.3 (2.2) | 12.7 (1.0) | 13.4 (1.2) | 15.4 (1.5) | ||
| Segmented leucocytes (%) | 0 | 48.5 (4.9) | 49.4 (4.3) | 49.6 (4.2) | 51.7 (7.1) | NS |
| 24 | 42.9 (2.1) | 44.1 (3.9) | 47.5 (5.1) | 49.1 (5.1) | ||
| Platelets (x 1000/μl) | T0 | 347.0 (38.4) | 472.7 (33.6) | 517.4 (46.7) | 446.0 (54.4) | NS |
| T24 | 404.7 (42.9) | 441.7 (33.2) | 530.9 (41.2) | 527.0 (54.7) |
Data are expressed as means (standard deviations). * Data compared using repeated measures analysis of variance ANOVA; NS: not significant.
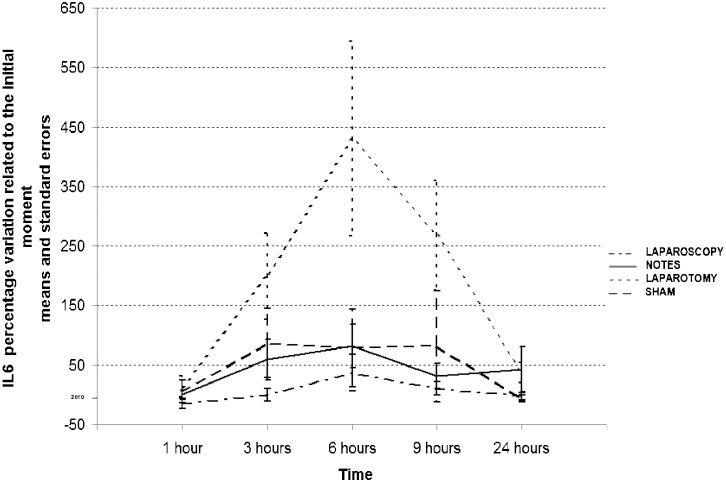
IL-1β, IL-10 and TNF-α levels were below the thresholds of detection for the respective assays and were not consistently detectable in serum samples. CRP measurements (Table 4) presented similar values for all groups at T0, with a significant increase in all groups at T24 (p<0.05) but with no statistically significant differences (p>0.05) among the groups. IL-6 was detected in study animals and presented different patterns in different groups (Table 5), Figure 1). In the laparoscopy, NOTES and sham groups, IL-6 values remained stable for 24 h, and there were no significant differences among the three groups. However, the laparotomy group displayed an increase in IL-6 levels between T1 and T3 that was statistically significant compared to the laparoscopy group (p<0.05). This increase was followed by a further increase between T3 and T6; at the latter time, a significant difference in IL6 expression was noted between the laparotomy group and all of the other groups (p<0.05). After T6, the IL-6 levels in these animals began to decrease; at T9, the laparotomy group presented a statistically significant difference in comparison with the laparoscopy and NOTES groups (p<0.05); and at T24, all groups presented similar patterns.
C-reactive protein measurements at T0 and T24.
| Sham | NOTES | Laparoscopy | Laparotomy | p-value* | |
|---|---|---|---|---|---|
| n = 6 | n = 12 | n = 8 | n = 8 | ||
| T0 | 44,913 (5035) | 38,460 (5847) | 46,786 (9552) | 48,195 (9856) | NS |
| T24 | 76,931 (5915) | 67,845 (6744) | 90,863 (16696) | 97,885 (9107) | |
| p-value* | <0.001 | ||||
T0: 0 min; T24: 24 h after T0. Data are expressed as means (standard deviations) in ng/ml. The data were compared using repeated measures ANOVA.
Interleukin 6 (IL-6) levels during the 24 h following the procedure.
| Group | Rates of IL-6 levels (%) compared to T0 | ||||
|---|---|---|---|---|---|
| T1 | T3 | T6 | T9 | T24 | |
| SHAM | 5.2 (18.7) | 86.7 (58.7) | 78.7 (65.2) | 81.3 (92.8) | -6.9 (5.5) |
| NOTES | 0.1 (8.0) | 59.2 (34.2) | 82.5 (36.5) | 31.2 (21.2) | 42.8 (37.9) |
| Laparoscopy | -14.6 (8.7) | -0.3 (10.7) | 36.6 (31.0) | 10.5 (10.9) | -3.0 (6.2) |
| LaparotomyD | 13.7 (18.0) | 199.3 (72.0)A | 430.1 (163.6)B | 268.0 (91.9)C | 37.1 (16.8) |
Data are expressed as means (standard deviations). A: Significant difference compared to the laparoscopy group at T3 (p<0.05); B: Significant difference compared to the laparoscopy, NOTES and SHAM groups at T6 (p<0.001); C: Significant difference compared to the laparoscopy and NOTES groups at T9 (p<0.05); D: Significant difference (p<0.05) within the laparotomy group: the difference (%) at T6 was higher than the differences (%) at T1, T3, T9 and T24; IL-6 differences (%) at T3 and T9 were higher than at T1 and T24.
It has been shown that laparoscopic surgery has less impact on the systemic inflammatory response than open surgery (7-13). With respect to NOTES, recent experimental studies using a porcine model to compare NOTES to laparoscopy with respect to the production of a systemic inflammatory response have produced conflicting results (14-19).
In our study, a significantly higher level of IL-6 was identified in animals treated with laparotomy than in the other groups. This finding permits us to infer that a more marked inflammatory response was present after laparotomy than after laparoscopy or NOTES. However, no significant difference in this parameter was noted between animals that received NOTES or laparoscopy; thus, based on observed levels of IL-6, both procedures resulted in the same intensity of inflammatory response.
In this experimental study, the acute inflammatory response induced by NOTES was compared to the response induced by laparoscopy by measurement of cytokine levels during and after the procedure. This comparison was conducted to encompass the known kinetics of these cytokines and to avoid secondary infection and sepsis, which could interfere with the results. The increase in CRP levels observed in all groups, the lack of any signs of peritonitis on necropsy and the presence of normal white blood cells (WBC) 24 h after surgery suggest that surgical trauma induced the inflammatory response with no secondary infection or sepsis.
Because of the need to schedule the surgical procedures, animals were assigned to their groups in a non-randomized manner. Nevertheless, the animals used in the study were similar in gender, weight, preoperative temperature, CBC, preoperative care protocol, protocols for sedation, anesthesia, ventilation, sample collection and postoperative care. Furthermore, the mean anesthesia times were similar among the four groups.
Anesthetic procedures are known to interfere with the inflammatory response (21,22). In a previous NOTES trial, Trunzo et al. (19) discussed the possible effects of non-standardization of the duration of anesthesia and suggested that a lack of standardization may have interfered with their results. Thus, in this experiment, an effort was made to standardize the duration of anesthesia so as to eliminate possible variations based on this factor.
The longer postoperative recovery observed for the laparotomy group compared to the NOTES and laparoscopy groups, which had similar postoperative recovery scores, reinforces the idea that laparoscopy and NOTES produced the same intensity of inflammatory response, as evidenced by IL-6 levels. It could be speculated that the degree of trauma induced by the procedures was insufficient to produce a systemic inflammatory response in the laparoscopy and NOTES groups. Nevertheless, considering that all groups presented significantly higher CRP levels at 24 h than at baseline, we can infer that surgical trauma in the NOTES and laparoscopy groups was sufficient to induce an inflammatory response.
An important point to consider is that in the NOTES group, a non-sterile, high-level-disinfected endoscope was used to access the peritoneal cavity through potentially contaminated oral and gastric cavities. Therefore, it would naturally be expected that, in contrast to laparoscopy, which is an aseptic procedure, NOTES would lead to greater levels of contamination and, consequently, a more marked inflammatory response, resulting in higher IL-6 levels. Another point to be considered is whether the changes in IL-6 levels observed in the NOTES group were caused by an inflammatory response or by secondary infection due to cavity contamination. The similar IL-6 curves observed for the laparoscopy and anesthesia groups suggest that the inflammatory response, rather than the presence of a secondary infection, was responsible for the typical curve. Furthermore, no signs of peritonitis were found in the NOTES animals.
Although no significant differences in CRP levels were observed among the experimental groups in this study (p>0.05), a significant difference in CRP levels at T0 and at T24 was observed for each individual group. These results contrast with previous reports that suggest that this biomarker presents low sensitivity in inflammatory response analysis (23). In our study, we observed highly variable results with no differences among the groups at T24. It is possible that the use of a maximal time of 24 h for collecting samples for CRP analysis may have interfered with the CRP results. While peak IL-6 levels are typically reached 4 to 6 hours postoperatively (24,25), it has been reported in the literature that the time required to reach peak CRP levels is between 24 and 48 h (13).
Genetic polymorphism can lead to differences in the inflammatory response among individuals, and such polymorphisms that are in part responsible for the large inter-individual variations in cytokine levels are observed in healthy individuals. Moreover, there are several technical difficulties associated with the use of cytokines and CRP as markers of the inflammatory response (5,26). Due to variability in the baseline cytokine levels observed in the animals in this study, we used the percentage difference from baseline to assess the biological effect of the experimental procedures being studied. McGee et al. (14) used a similar approach in the analysis of their results (5,26). In our study, IL-1β, IL-10 and TNF-α were not consistently detectable in serum samples. Other authors have reported similar analytical difficulty (14). In contrast, in a randomized trial in which TNF-α and IL-1β were used to assess the inflammatory response, Bingener et al. (16) were able to measure IL-1β and TNF-α in 94% and 82% of the experimental subjects, respectively. The authors of that study also reported a statistically significant difference between NOTES and laparoscopy, with higher levels of TNF-α on postoperative day 7 for NOTES compared to laparoscopy. This finding differs from the findings of McGee et al. (14), who reported a small increase in TNF-α levels in NOTES-treated animals on postoperative day 14.
Most recent studies comparing NOTES and laparoscopy in porcine models have reported that IL-6 is below detectable levels in most samples collected. However, based on the levels of measurable cytokines such as TNF-α, the inflammatory response following NOTES was not significantly different from the response following laparoscopy in these studies (27,28). It is possible that conflicting results on inflammatory responses secondary to NOTES could be due to technical limitations associated with the determination of cytokine levels and/or the use of different experimental porcine models.
Increased hematocrit levels were observed in all groups in our study, possibly because of hemoconcentration. Leucocyte count is reported to be a very sensitive marker for the identification of complications related to infection (29). In our study, total leucocyte counts were stable at T24 in all groups, indicating the absence of secondary infection during the experiment. In relation to platelet count, only the animals in the NOTES group presented a slight decrease, with no significant differences among groups. It is possible that the relative thrombocytopenia observed in the NOTES animals in our study and also observed by Bingener et al. (15) could be secondary to the occurrence of bacteremia during the procedure. This hypothesis should be investigated in further studies.
Regarding the technical aspects of NOTES, gastric closure with no fistula is a prerequisite in inflammatory response studies designed to compare NOTES with laparoscopy and is mandatory in human trials (1,2). Several methods have been proposed for gastrostomy closure in the porcine model, including suture, clips and the use of a submucosal tunnel or a technically simple closure device (1,2,20). In our study, transgastric access to the peritoneum was performed via a submucosal tunnel that was modified using a hot biopsy forceps for dissection (20). Despite its greater difficulty compared with simple puncture of the gastric wall, use of the submucosal tunnel was, in our opinion, very important as an auxiliary mechanism for gastrostomy closure. We observed that closure by means of clips leads to mucosal apposition, which is likely neither sufficient nor safe in NOTES procedures. Furthermore, when gastrostomy caused significant mucosal edema, the endoscopic clips were unable to grasp and approximate the edges of the gastrostomy, as reported by Sood et al. (28), resulting in inefficient closure. In these cases, an alternative closure method involving the injection of 0.5 ml of diluted cyanoacrylate inside the tunnel was performed. The leakage test was negative in all animals, showing that the submucosal tunnel is an effective auxiliary technique for gastrostomy closure in transgastric NOTES. It is possible to postulate that the submucosal tunnel, which has two holes at different levels, could function as a valve-like mechanism, avoiding gastric fistula and contamination of the peritoneal cavity. This hypothesis should be evaluated in further studies.
Another technical point to consider is the role in the inflammatory response of the gas insufflation used to create pneumoperitoneum. In our experiment, room air was used for NOTES, and carbon dioxide (CO2) was used for laparoscopy. It has been shown that CO2 promotes an acidic environment that can modulate the inflammatory response in laparoscopy (30). However, as demonstrated by Trunzo et al. (19), the type of gas used (CO2versus air) to create the pneumoperitoneum did not affect the cytokine profile after NOTES, and CO2 pneumoperitoneum had no apparent immunomodulatory effect.
In conclusion, the findings presented here, which demonstrate that there are similar systemic inflammatory responses after NOTES and laparoscopy and concur with the results of a study conducted by Narula et al. (31), advance the progress of research on NOTES and help to establish a foundation for future research in humans.
ACKNOWLEDGMENTSThis work was partially supported by the São Paulo Research Foundation (FAPESP), Brazil.
Rezende M and Della Libera E, who contributed equally to this work, conceived and designed the study and wrote the paper. Rezende M, Della Libera E, Rodrigues R and Gomes G handled the animals and collected data. Rezende M, Salomão R and Brunialti M conducted the laboratory analyses. Rezende M, Montero EF and Della Libera E analyzed and interpreted the data. Della Libera A, Montero EF, Salomão R and Ferrari A critically revised the paper for important intellectual content.
No potential conflict of interest was reported.