Blood neurotrophins, such as the brain-derived neurotrophic factor, are considered to be of great importance in mediating the benefits of physical exercise. In this study, the effect of acute strength exercise and the involvement of small versus large muscle mass on the levels of plasma brain-derived neurotrophic factor were evaluated in healthy individuals.
METHODS:The concentric strengths of knee (large) and elbow (small) flexor and extensor muscles were measured on two separate days. Venous blood samples were obtained from 16 healthy subjects before and after exercise.
RESULTS:The levels of brain-derived neurotrophic factor in the plasma did not significantly increase after both arm and leg exercise. There was no significant difference in the plasma levels of the brain-derived neurotrophic factor in the arms and legs.
CONCLUSION:The present results demonstrate that acute strength exercise does not induce significant alterations in the levels of brain-derived neurotrophic factor plasma concentrations in healthy individuals. Considering that its levels may be affected by various factors, such as exercise, these findings suggest that the type of exercise program may be a decisive factor in altering peripheral brain-derived neurotrophic factor.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophic factor family that plays key roles in regulating survival, growth and maintenance of neurons.1 Animal and human research has shown that exercise increases neuronal survival and resistance to brain insult,2,3 promotes brain vascularization, stimulates neurogenesis, enhances learning and contributes to maintenance of cognitive function, all of which are modulated by BDNF.2 Exercise for several days enhances BDNF production in the hippocampus and other areas of the central nervous system in rats, which suggests that exercise induces neurotrophin release (please see4 for a review).
Although it is well known that BDNF synthesis is centrally mediated and activity dependent and that exercise increases BDNF transcription in the brain,5 recent findings have reported that physical exercise increases circulating BDNF levels in healthy humans.6-8 The main source of circulating BDNF at rest and in response to exercise has not been defined. Nevertheless, Tang et al.9 proposed that platelets provide the increased source of serum BDNF in response to exercise. BDNF is also increased in plasma samples after exercise, which suggests that BDNF might originate from several other cell sources. Researchers have demonstrated that the brain and/or vascular endothelium produce BDNF during prolonged endurance exercise.10,11 An increase in BDNF during ramp tests to exhaustion has been observed,8 and the magnitude of its increase is exercise intensity dependent.7
While the effects of moderate (aerobic) and intense exercise on blood BDNF have been demonstrated,6-9 little information about the effect of strength exercise on BDNF has been reported. A few studies on this topic have investigated the effects of strength exercise on blood plasma concentrations of BDNF and Insulin-like growth factor 1 in humans.12,13 Despite the functional adaptations that were induced by strength training in these studies, a single strength session13,14 or strength training12,13 was insufficient to induce adaptations in basal BDNF plasma concentrations. In opposition to the above findings, Yarrow et al.14 reported increases in serum BDNF levels after resistance exercise. Although a single strength session was not able to significantly alter BDNF levels, we hypothesize that the response of BDNF to strength exercise may differ depending on the type of exercise. A number of reports in healthy human subjects suggest that the magnitude of the circulatory responses to exercise is dependent on the size of the active muscle mass.15-19 Along these lines, we investigated the effects of acute strength exercise (isokinetic muscle contraction) on plasma BDNF concentrations in healthy individuals and compared the involvement of small versus large muscle mass on blood BDNF concentrations.
MATERIALS AND METHODSSixteen young, healthy male subjects (age: 26.4 ± 3.7 yr; height: 176.5 ± 7.6 cm; weight: 79.9 ± 13.9 kg; reported as the mean ± SD) were recruited for the study, which was approved by the ethics committee of the Universidade Federal de São Paulo (UNIFESP). The volunteers were informed about the aims of the study and received written, detailed explanations about the experiments, the potential discomforts, the risks and the procedures employed in the investigation. Subjects with known cardiovascular or metabolic disorders or orthopedic limitations, as established by the American College of Sports Medicine, were excluded from the study.20 Therefore, all volunteers were in good health and free of any physical condition that would place them at risk during participation in the study. The participants were not previously involved in regular physical activities. All subjects signed an informed consent document. After signing the consent form, the Beck Depression Inventory and Trait and State of Anxiety (IDATE) were given to the volunteers to exclude subjects with signs of depression or anxiety.
The muscular strengths of concentric knee and elbow flexor and extensor muscles for both dominant and non-dominant sides were measured on separate days (knee muscles were tested on day 1, and elbow muscles were tested on day 2) using a Cybex 6000 isokinetic dynamometer (Ronkonkoma, NY). The dynamometer was calibrated before testing each subject. The Cybex 6000 was arranged according to the guidelines described in the user's manual for measurement of knee and elbow flexion/extension actions. The flexor and extensor muscles of the dominant and non-dominant knees were assessed in the seated position with the hips flexed at 85 degrees. The range of motion was set at 110 degrees, considering full extension as 0 degrees. One week later, flexor and extensor elbow muscles were tested in the supine position with the shoulder abducted at 45 degrees in the frontal plane and the forearm positioned in the neutral position. The range of motion was set at 130 degrees, considering full extension as 0 degrees. Exercise protocols were the same for knee and elbow muscles. Five series of ten repetitions at 60 deg/s were performed after a 5-min warm-up on a stationary bicycle. The dominant side was tested first in all subjects. Successive series were separated by 40 sec of rest, and the non-dominant knee was examined 2 min after the complete trial of the dominant side. A gravity correction was performed in which the volunteers were asked to perform a series of maximal knee and elbow contractions. Visual feedback from the computer screen was not allowed. Heart rates (HR) were recorded for each subject both pre- and post-exercise. The exercise duration for each knee and elbow was as follows: the time duration of each set (10 repetitions) for one knee was approximately 30 sec. The total duration of exercise, including the rest period (40 sec between sets), was 5.10 min. The duration of each set (10 repetitions) for one elbow was 43 sec. The total duration of each exercise, including the rest period (40 sec between sets), was 6.15 min.
Blood draws were performed at 1 min post-exercise and after a 30-min rest period. Blood samples were taken from antecubital veins, placed into plasma venipuncture tubes, separated by centrifugation (3000 rpm for 10 min at 4 °C) and stored at −70 °C until use. Plasma BDNF concentrations were analyzed by enzyme immunoassay using ELISA (Enzyme-linked immunosorbent assay) kits by Millipore (Temecula, CA, USA) according to the manufacturer's description. Statistical analyses were performed by employing ANOVA (Analysis of Variance) with a Tukey's post-hoc test. A p-value < 0.05 was considered to indicate statistical significance.
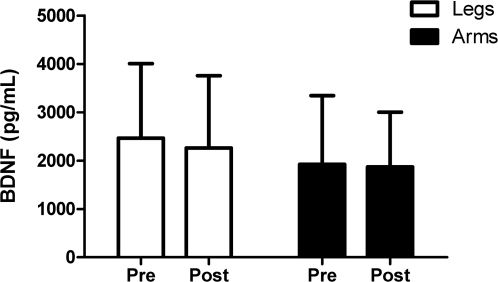
RESULTSAll volunteers completed the exercise protocols. Plasma BDNF did not significantly increase after both arm and leg protocols (legs: from 2464±1543 pg/ml at rest to 2261±1493 pg/ml after exercise; arms: from 1925±1417 pg/ml at rest to 1870±1131 pg/ml after exercise; all values are reported as the mean ± SD) (Figure 1). No significant difference in the plasma concentration of BDNF was observed after arm and leg exercises. Analysis of the HR response after strengthening exercises showed a significant increase immediately after exercise and at 1 min post-exercise compared to the values at rest (p<0.001). Individual BDNF values (Table 1) and variations in HR (Table 2) pre- and post-exercise are presented below.
Plasma BDNF concentration (pg/ml) pre- and post-isokinetic strength exercise.
| Legs | Arms | |||
|---|---|---|---|---|
| Subject | Pre | Post | Pre | Post |
| 1 | 1965 | 2170 | 1200 | 2515 |
| 2 | 1370 | 1160 | 2945 | 1410 |
| 3 | 3505 | 2335 | 1790 | 1650 |
| 4 | 1825 | 2175 | 2840 | 3050 |
| 5 | 2350 | 1390 | 665 | 1310 |
| 6 | 3860 | 5055 | 6365 | 4980 |
| 7 | 2180 | 2035 | 1225 | 2785 |
| 8 | 967 | 935 | 2900 | 1175 |
| 9 | 1355 | 805 | 780 | 820 |
| 10 | 6630 | 2785 | 1605 | 2115 |
| 11 | 3235 | 5650 | 2035 | 1960 |
| 12 | 1175 | 1285 | 1865 | 2285 |
| 13 | 4587 | 1498 | 955 | 855 |
| 14 | 1365 | 1525 | 710 | 1145 |
| 15 | 1220 | 1025 | 2050 | 1690 |
| 16 | 1841 | 4350 | 870 | 173 |
| Mean | 2464 | 2261 | 1925 | 1870 |
Heart rate values at rest, immediately after and at 1 min post-isokinetic strengthening exercise in eight male subjects. Heart rate measurements were taken after the second set of contractions from the legs and arms.
| Legs | Arms | |||||
|---|---|---|---|---|---|---|
| Subject | Rest | 0 min post- exercise | 1 min post- exercise | Rest | 0 min post- exercise | 1 min post- exercise |
| 1 | 85 | 146 | 134 | 77 | 144 | 118 |
| 2 | 82 | 125 | 90 | 80 | 140 | 110 |
| 3 | 85 | 171 | 129 | 80 | 142 | 89 |
| 4 | 88 | 162 | 137 | 94 | 130 | 106 |
| 5 | 95 | 163 | 131 | 76 | 136 | 107 |
| 6 | 76 | 147 | 104 | 79 | 130 | 105 |
| 7 | 90 | 160 | 125 | 87 | 151 | 120 |
| 8 | 84 | 140 | 108 | 76 | 104 | 95 |
| Mean | 85.6 | 151.8 ∗ | 119.8 ∗∗ | 81.1 | 134.6 ∗ | 106.3 ∗∗ |
| SD | 5.6 | 15.0 | 17.0 | 6.3 | 14.3 | 10.5 |
The present results demonstrate that acute strength exercise does not induce significant alterations in BDNF plasma concentrations in healthy individuals. Based on previous reports that showed elevated blood BDNF after moderate (aerobic) and intense exercise,6-9,11 we would expect that strength exercise might also increase plasma BDNF levels. However, there were no significant differences in the levels of plasma BDNF during arm and leg exercises. One study that used a strength training protocol to evaluate peripheral BDNF analyzed the effects of “strength training” with high loads on blood plasma concentrations of BDNF and IGF-1 (insulin-like growth factor) in humans.12 Importantly, these investigators evaluated basal plasma BDNF concentrations and not BDNF levels after intensive effort. Subsequent studies demonstrated that a single strength session did not induce significant changes in blood BDNF levels.13,14 To the best of our knowledge, this is the first study that compares the influence of large or small muscle groups on BDNF plasma concentrations in sedentary, healthy humans using a protocol of acute strength exercise.
To justify our findings, it is important to understand several factors that may affect peripheral BDNF levels. Reports have shown that various features of exercise stimuli, including intensity, duration and mode of activity, can affect BDNF levels. Protocols of moderate or intense exercise have been shown to increase levels of BDNF in the blood. For instance, Gold and co-workers6 showed that their moderate exercise task (30 min of bicycle ergometry at a maximum of 60% VO2) caused a transient increase in serum BDNF levels. Serum BDNF was not significantly induced after exercise performed at low intensity (20% below ventilatory threshold), but it was enhanced after 30 min of intense exercise (10% above ventilatory threshold).7 Correspondingly, results from Tang and collaborators9 showed a significant, acute increase in serum BDNF levels after 25 min of step-exercise. Interestingly, short periods (10 min) of moderate exercise did not change serum BDNF levels, but a very transient increase in serum BDNF levels was observed during a ramp test to exhaustion.8
As mentioned above, the degree of physical effort during the exercise protocol may be important for altering blood BDNF levels. Along these lines, HRs were monitored during and after exercise in our study. HR increased significantly after exercise, but it was not substantially different from the post-exercise HR values reported in the majority of studies7-9,12 Therefore, at the moment of blood collection (1 min post-exercise), HRs were not greatly elevated compared to other reports. In our work, an intermittent strength protocol with a shorter duration of execution was used, which does not permit comparison of our BDNF values with those in the literature. Protocols of strength exercise with longer duration need to be tested to confirm whether exercise duration, independent of the type of exercise, is a determining factor in altering BDNF. Additionally, in light of the massive motor recruitment after strength exercise (i.e., maximal muscle contractions), we expect that plasma BDNF might be increased. This idea is supported by reports in rodents that indicate that BDNF mRNA increases in skeletal muscle in response to contraction,21,22 suggesting that the source of BDNF is neurons within the skeletal muscle beds. On the other hand, Matthews et al.23 showed that although BDNF is produced by the muscle, it is not transported into the circulation. To this end, we verified whether the type of exercise (strength work using large versus small muscle mass) in our study could activate both peripheral (recruitment of great motor units) and central (motor cortex) BDNF. Neither strength exercise nor activation of large versus small muscle groups was able to alter peripheral BDNF levels (although we cannot discriminate between peripheral and central blood BDNF concentrations in our study). Contrary to the concept that a larger muscle mass could stimulate greater physiological and biochemical changes, no significant variations were observed.
These preliminary results should be interpreted with caution. In humans, the BDNF response to exercise differs depending on the type and intensity of exercise. Thus, strength exercise can induce a different metabolic response compared to aerobic exercise, which is manifested by lower oxygen consumption despite higher levels of muscular exertion. An alternative hypothesis is presented by Rasmussen and collaborators10, who found that the brain is a major contributor of circulating BDNF following exercise. In conclusion, these findings suggest that the mode of the exercise program can be a decisive factor in altering peripheral BDNF. Knowledge about the effects of exercise on concentrations of blood neurotrophins will therefore help to optimize the health benefits of exercise, and further intervention studies using other strength exercise protocols are necessary.