The purpose of this study was to describe the probable mechanism of the volume increase of laparoscopically harvested omentum flaps used to treat breast deformities.
METHODSA histological analysis of omentum samples was performed to study the volume increase of laparoscopically harvested omentum flaps. Samples were harvested immediately after the transposition of the omentum from the abdominal cavity to the breast region and during the second surgical procedure for breast symmetrization of eight patients submitted to the transposition of the omentum flap. Changes in the morphometric measurements of the adipocytes (perimeter, diameter, and area), microvascular density (as measured by the CD31 endothelial marker), and immunohistochemical expression of VEGF were documented.
RESULTSThe increases in adipocyte size and microvascular density were statistically significant (P ≤ 0.012). The expression levels of VEGF were lower in the second set of samples when compared to the first set, but the differences were not statistically significant (P < 0.093).
CONCLUSIONThese results demonstrate an increase in cellular volume as measured by adipocyte perimeter, diameter, and area. Moreover, the increase in the number of vessels in the second set of samples suggests that neoangiogenesis was stimulated by the initial increase in VEGF expression levels observed in the first set of samples. The increase in VEGF expression in the flap may have been caused by adipocyte hypertrophy resulting from neoangiogenesis.
There are numerous reports of tissue volume increase in human1,2 and animal (rodents, bovines, swine, etc.)3-5 tissues, and in cell cultures.6,7 However, such increases have never been observed in any other adipose flaps.8,9
The omentum is composed of highly vascularized fatty connective tissue; it is approximately 25×35 cm long, its volume varies according to the size of the patient but it is not always predictable.10 The use of the omentum for various purposes has been reported,8-14 because of its peculiar structure, its rich vascular supply that enables its high capacity of absorption, its pronounced angiogenic activity that supports local (and ischemic) tissues, its innate immune function, its ability to adhere to local structures and its high concentration of “tissue factors” that promote hemostasia.8,11–14 It may promote angiogenic activity in structures adjacent to where it is applied to in animals15 and in human beings.11
The omentum is predominantly composed of mature adipocytes that do not multiply and that represent an important share of visceral fat in the human body. It contains adult stem cells and progenitor cells or preadipocytes, which are smaller and able to differentiate.16 Adipocytes are now seen as energy storage cells as well as cell with endocrine function.17 The observation that they secrete leptin established that adipose tissue is an endocrine organ communicating with the central nervous system.18 Hypoxic adipocytes express more angiogenic factors, suggesting that hypoxia might be one of the modulators of the angiogenic process.19-21
Zhang et al in 1997, suggested that the vascular endothelial growth factor (VEGF) is probably the main angiogenic factor produced by the omentum.3 Increased VEGF expression by hypoxic omentum cells may be responsible for increased omentum angiogenic activity under ischemic conditions through paracrine regulation. The trauma of surgically transplanted omentum induces increased VEGF expression, similar to that induced by hypoxia.3,5
Adipose tissue growth can be controlled by local vascularization. Adipocyte development and vascular morphology are dependent on triglyceride deposit whereas adipocyte size does not depend on storage volume.5 Increased triglyceride induces preadipocytes to become mature adipocytes with consequent hypertrophy and hyperplasia. The increase in the bulk of adipocytes is accompanied by an increase in the microvascular network22 and, conversely, neovascularization accompanies adipose tissue growth. Evidence suggests that the O2-sensitive signaling mechanism regulates adipogenesis.19 However, preadipocyte differentiation is inhibited by hypoxia leading to adipocyte hypertrophy without hyperplasia.23
Measurement of VEGF, neoangiogenesis by the CD31 (cluster of differentiation molecule) or PECAM-1 (Platelet Endothelial Cell Adhesion Molecule) together with morphometric measurements of the adipocyte represent adequate means to investigate, clarify, and document the increase in postoperative volume of the omentum flap.24-26
In this project we used laparoscopically harvested omentum flaps to treat breast deformities, with a significant volume increase of the omentum over the first months following its transposition. Our main purpose here was to establish whether the volume increase of the omentum flap transposed to the breast region is caused by adipocyte hypertrophy or hyperplasia. This is the first “in vivo” documentation of the direct effect of neoangiogenesis in human adipose tissue, as evidenced by modification of volume observed between two consecutive surgical procedures.
METHODSIn order to assess the nature of the apparent volume increase of the omentum when transposed to the breast, eight patients who underwent this procedure were studied.
Variables under StudyChanges in size of the adipocyte were studied by measuring the perimeter, diameter and area of adipocytes. The microvascular density was estimated by the counting of vessels stained by the CD31 endothelial marker and in the immunohistochemical expression of VEGF.
Study DesignThis was an intragroup experiment (within subjects design) which included omentum samples obtained at the first and second surgical procedures performed on five patients with cancer and three with congenital malformation of the breast. The patients were referred to the clinic for reconstructive surgery, according to the criteria described below. The procedures were carried out in hospitals located in the city of Porto Alegre between 2005 and 2010 and all were operated on by the same surgical team.
CriteriaThe experiment included patients who underwent laparoscopically harvested omentum flap transposition to correct deformities resulting from the treatment for breast cancer or congenital deformities of the breast, at two surgical procedures. The patient's body mass index (BMI) was stable between the first and the second surgical procedures, showing no increase greater than 1 kg/m2. Cases of contraindication to laparoscopic procedure, previous abdominal surgery which removed or compromised the viability of the omentum, previous inflammatory process of the abdominal cavity, patient refusal to participate in the study, and variation of BMI greater than 1 kg/m2 between the two surgical procedures were exclusion criteria.
Surgical TechniqueThe omentum flap was laparoscopically harvested and transposed to the region of the deformity of the breast, pedicled to the Right Gastroepiploic Artery and vein, as previously described by Costa et al.8,9
In order to mobilize the flap, it was necessary to reduce significantly the blood supply, allowing flow to run only through one of the Gastroepiploic Arteries and not through the short Gastric Arteries, which would have caused temporary hypoxia.
Omentum samples were obtained at the first surgical procedure, shortly after its transposition from the abdominal cavity to the breast region, and at the second surgical procedure during the complementary treatment for breast symmetrization, at variable times, depending on individual circumstances such as the will of the patient, her availability, and the clinical conditions for a second surgical procedure.
Tissue SamplesSamples measuring one square centimeter were obtained at the time of the omentum flap transposition to the breast region and at the time for complementary reconstruction. Immediately after collection, fragments of tissue were fixed in 4% buffered formalin solution for 24 hours. The material was subjected to routine histological processing, immersion in graded alcohol solutions, clearing in xylene, and paraffin impregnation. Four-micron-thick sections were stained with hematoxylin-eosin and morphometric evaluation was conducted. Two additional cuts were collected on slides previously prepared with organosilane and subjected to the immunohistochemistry technique, using antibodies against VEGF (Clone VG1, Dako, Carpinteria, CA, USA) and CD31 (clone JC70A, Dako, Carpinteria, CA, USA).
The primary antibodies were incubated for twelve hours at a temperature of 4oC, at dilutions of 1:50 (VEGF) and 1:40 (CD31), followed by the application of the streptavidin horseradish peroxidase conjugate (LSAB, Dako), and diamino-benzidine tetrahydrochloride (Kit DAB, Dako). A negative control was obtained by omission of the primary antibody.
Each of the slides stained with hematoxylin-eosin was scanned by an experienced pathologist, blinded to clinical and surgical data, by means of a Zeiss Axiolab microscope, equipped with a digital camera connected to a computer, using 10 high-power fields (400X). A morphometric study was performed using Image Pro Plus software (Media Cybernetics), and the measurement of the maximum perimeter and diameter of adipocytes was obtained in micrometers (μm); the measurement of the area of adipocytes was obtained in square micrometers (μm2). The corresponding means of each sample for each individual patient were calculated.
Microvessel density was obtained by counting vascular structures stained by CD31 antibody in images captured at medium magnification (200X), also calculating the corresponding means of each sample for each individual patient.
To study the immunohistochemical expression of VEGF, the resulting segmented brown color, corresponding to the Immunohistochemistry VEGF expression was quantified by VEGF colored pixels per image (pixels/image).
All patients had their BMI observed in the interval between the first and the second surgery.
Statistical AnalysisThe quantitative data obtained in the measurements were summarized by median values and minimum and maximum values. Comparison of procedures 1 and 2 was performed by nonparametric Wilcoxon test. The level of significance was 5%. Data were analyzed using SPSS 17.0. All patients signed an informed consent form and the project was approved by the Ethics Committee of the Federal University of Rio Grande do Sul, No. 19749.
RESULTSEight patients were included in the study. In all the patients a visible volume increase of the breast was observed (Figure 1).
Five of the selected patients were being treated for breast cancer and three were being treated for benign breast disease. The average age was 45 (20–60). Table 1 shows some variability in age, and the difference between the youngest patient and the oldest patient is practically three generations. Furthermore, it has been observed that the time interval between procedures 1 and 2 varied substantially. The average time interval between the two samples was 192 days (74–320).
Demographic data and patient statistics (n = 8).
| Surgery 1 | Surgery 2 | ||||||
|---|---|---|---|---|---|---|---|
| Patient | Age (years) | Height (m) | Weight (Kg) | BMI (Kg/m2) | Weight (Kg) | BMI (Kg/m2) | Interval* (days) |
| A | 23 | 1.64 | 49 | 18.2 | 49 | 18.2 | 74 |
| B | 51 | 1.55 | 49 | 20.4 | 51 | 21.2 | 320 |
| C | 55 | 1.65 | 60 | 22.0 | 59 | 21.7 | 162 |
| D | 50 | 1.58 | 56 | 22.4 | 56 | 22.4 | 197 |
| E | 48 | 1.59 | 60 | 23.7 | 61 | 24.1 | 301 |
| F | 60 | 1.57 | 62 | 25.2 | 61 | 24.8 | 140 |
| G | 20 | 1.69 | 60 | 21.0 | 57 | 20.0 | 183 |
| H | 53 | 1.51 | 57 | 25.0 | 58 | 25.4 | 162 |
| Mean | 45 | 1.60 | 57 | 22.2 | 57 | 22.2 | 192 |
| SD | 15 | 0.06 | 5 | 2.4 | 4 | 2.5 | 82 |
| Minimum | 20 | 1.51 | 49 | 18.2 | 49 | 18.2 | 74 |
| Maximum | 60 | 1.69 | 62 | 25.2 | 61 | 25.4 | 320 |
SD: Standard Deviation, BMI: Body Mass Index.
Comparison between BMI1 x BMI2: P = 0.916
Individual BMI was stable during the period between the first and the second surgical procedure when samples were collected, and upper individual variation was not greater than 1 kg/m2 (P = 0.916). Weight gain above this limit was an exclusion criterion to avoid confusion between omentum volume increase and weight gain (Table 1).
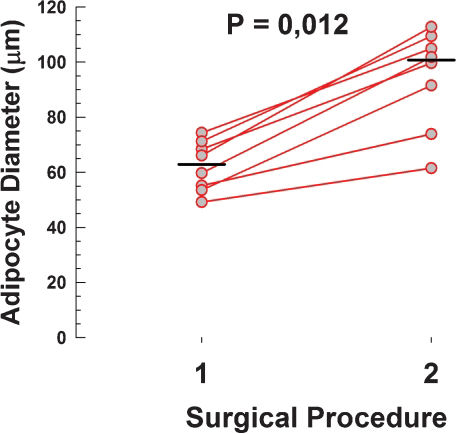
Table 2 shows a summary of morphometric measurements, vascular density and intensity of VEGF expression per field. All measurements between surgery 1 and surgery 2 showed an increase in value. The measurements of perimeter (Figure 2), diameter (Figure 3), area (Figure 4) and vascular density (Figure 5) – increased from the first to the second sample and reached statistical significance (P ≤ 0.012). VEGF (Figure 6), however, was higher in the first sample compared with the second, but the reduction did not reach significance (P <0.093). One patient had a reverse result of VEGF, i.e. increased in the second sample, which can be attributed to biological variability or additional stimulus not registered by the observer (Table 2 and Figure 6).
Morphometric measurements, vascular density and VEGF of the patients (n = 8).
| Perimeter (μm) | Diameter (μm) | Area (μm2) | Vessels (n°/field) | VEGF (Kpixels/field) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| A | 164 | 200 | 49 | 61 | 1850 | 3103 | 5.5 | 13.8 | 96.3 | 84.7 |
| B | 176 | 188 | 55 | 74 | 2502 | 4306 | 6.0 | 15.5 | 221.7 | 156.8 |
| C | 193 | 291 | 54 | 92 | 2308 | 6201 | 5.1 | 16.6 | 46.3 | 81.9 |
| D | 240 | 372 | 74 | 105 | 3219 | 9875 | 5.0 | 16.6 | 89.7 | 64.4 |
| E | 224 | 298 | 68 | 100 | 3789 | 6710 | 5.6 | 15.2 | 59.9 | 41.9 |
| F | 230 | 354 | 71 | 109 | 4019 | 9207 | 4.8 | 16.4 | 55.2 | 36.8 |
| G | 198 | 227 | 60 | 102 | 2798 | 8463 | 4.6 | 16.4 | 109.7 | 91.4 |
| H | 193 | 384 | 66 | 113 | 3929 | 10504 | 5.8 | 17.4 | 96.3 | 49.9 |
| Median | 196 | 295 | 63 | 101 | 3009 | 7587 | 5.3 | 16.4 | 93.0 | 73.2 |
| Minimum | 164 | 188 | 49 | 61 | 1850 | 3103 | 4.6 | 13.8 | 46.3 | 36.8 |
| maximum | 240 | 384 | 74 | 113 | 4019 | 10504 | 6.0 | 17.4 | 221.7 | 156.8 |
| P | 0.012 | 0.012 | 0.012 | 0.011 | 0.093 | |||||
VEGF: Vascular Endothelial Growth Factor, Kpixels: pixelsX 1000
1: first surgical procedure, 2: second surgical procedure
Thus, it can be said that these results indicate an increase in cell volume (Figure 7A and B) which was consistent when three different measurements were obtained: perimeter, area, and diameter of adipocytes. Moreover, the increase in the number of vessels in the second sample (Figure 7C and D) suggests neoangiogenesis stimulated by the initial increase in VEGF values, as documented in the first sample.
On the top, VEGF immunohistochemical expression shows no noticeable difference between the initial moment (A) and the second moment (B) (100X original magnification); but the adipocyte sizes are visible bigger in the second moment. In the second line, CD31 highlights endothelial lining. There are few vessels at the fist moment (C) and more vessels at the second moment (D) (200X original magnification).
Laparoscopycally harvested omentum flaps were used to treat breast deformities, with significant volume increases, which, to the best of our knowledge has never been reported in any other adipose flap. This volume increase was observed in all of the patients during the first 3 to 5 months following transposition. At the moment of the transposition of the omentum flap to the region of the breast, the adipose tissue was submitted to a transitory hypoxia, which might conceivably have stimulated VEGF and, consequently, neoangiogenesis, leading to the overall increase of the flap volume. This increase might be attributed to cell proliferation (adipocyte hyperplasia) or to increased cell size (adipocyte hypertrophy). It is very probable that the high initial VEGF was responsible for the observed effect, by inducing increased vascularization or neoangiogenesis; this was confirmed by the increased number of vessels per field in the second sample, similar to what was reported in rabbits by Zhong et al.22 In Zhong's study the new vessels disappeared after three months of observation. We have observed a maintained increase in vessel density nearly a year after omentum transposition; this however cannot be explained by the hypoxia, which was only temporary during the perioperative tempo. No explanation can be offered for this maintained neovascularization, nor for for the increased omentum volume in all of these patients. The analysis of VEGF variation between the samples is marred by a confounding factor, namely the lack of significance of the reduction of values between surgery 1 and 2. This however may have been caused by the punctual case of patient “C” who exhibited a paradoxical increase in VEGF value. This case is analyzed in Figure 6.
The difference in time intervals between the first and second surgeries (74 to 320 days) and the age heterogeneity of the patients (20 to 60 years) in this study, may be prima facie interpreted as a limitation of the research; however, it turned out to be of particular interest for making it possible to highlight the consistency of the supposed effect of VEGF stimulation, resulting in neoangiogenesis and hypertrophy of the adipocytes in all the patients.
According to the Bradford Hill criteria, the important diversity of ages among patients along with the interval between the two procedures reinforces the consistency of the effect of this study because, in spite of the individual procedural variations, the same effect occurred.27
In this study, evidence showed the extend to which changes in the tissue microenvironment, by temporary hypoxia produced by the mobilization of the omentum flap, are able to produce a cellular response, which in this case is neoangiogenesis and adipocyte hypertrophy.
CONCLUSIONThis study was carried out based on the clinical observation of the increase in the volume of the omentum flap which occurred as a spontaneous post-operative evolution, a phenomenon never before induced “in-vivo” in human beings. It was probably caused by adipocyte hypertrophy resulting from neoangiogenesis, induced by increased VEGF resulting from temporary hypoxia. Complementary studies are needed to investigate why neovascularization and omentum volume is maintained for a long time after the triggering factor stops, a response unlike what happens in other adipose tissues.
Conflict of Interest Disclosure: None of the authors had any financial or personal conflict of interest.