Critically ill patients frequently require prolonged endotracheal intubation and ventilatory support.1 Several studies have investigated the incidence of anatomic laryngotracheal injury after extubation.2-5 Studies on functional alterations are rare, although dysphagia and dysphonia are the most frequent clinical symptoms after extubation.6,7 Swallowing dysfunction and pulmonary aspiration often occur in patients who receive prolonged mechanical ventilation.7-9 After the weaning period and extubation, patients are at risk of aspiration due to the possible residual effects of pharmacological sedation, alterations in upper airway sensitivity that relate to prolonged endotracheal intubation, and the presence of the nasogastric tube.10 These studies have reported that aspiration may lead to severe complications, such as transient hypoxemia, mechanical obstruction with atelectasis, bronchospasm, chemical pneumonitis, or pulmonary infection;11,12 however, the incidence of swallowing dysfunction and the rate of resolution of swallowing abnormalities have not been well defined.13,14
Swallowing is a complex sensorimotor function that depends on the integrity of the mechano- and chemo-receptors for the sequential stimulation and inhibition of the upper aerodigestive tract; this process must occur in a coordinated and rapid form for the transport of foods and liquids through the mouth and pharynx to the esophagus.15
During swallowing, the closure of the larynx and the respiratory pause during swallowing are vital protective mechanisms that prevent aspiration. Airway protection primarily occurs with the elevation and anteriorization of the larynx toward the hyoid bone and with larynx closure at three levels: the true vocal folds adduct to close the glottis, the false vocal folds adduct, and the arytenoids anteriorly fold against the epiglottis to close the laryngeal aditus.16
There is a temporal and physiological correlation between breathing and the components that are involved in the protection of the airway during swallowing; however, the contribution of these alterations to the occurrence of pneumonia remains unknown,17 primarily due to the technological limitations of assessing objective measures (qualitative and quantitative) in the evaluation of serious patients at the bedside in intensive care units.18
Functional studies and the use of new technologies are necessary to understand the pathophysiology of the respiration–swallowing interaction, which are fundamental to the early detection of aspiration so as to prevent pulmonary aspiration, standardize research, and standardize the rehabilitation of patients who are submitted to mechanical ventilation (MV) and prolonged intubation after extubation in intensive care units.
The purpose this article is to introduce the use of non-invasive and portable technologies that can be used at the bedside for the evaluation and combined recording of respiration–swallowing interactions in the intensive care environment. Specifically, these non-invasive and portable technologies include infrahyoid muscle surface electromyography (sEMG), swallowing accelerometry (piezoelectric sensor), and inductive respiratory plethysmograph (RIP).
METHODSThis technical preliminary study was performed in two participants: a healthy male volunteer (27 years) without a history of respiratory or swallowing disease and a male patient (22 years) who was admitted to the respiratory intensive care unit and who required four days of intubation and mechanical ventilation due to respiratory failure. Both subjects provided written informed consent, which was approved by the local Ethics Committee.
The patient was evaluated 48 h after extubation under stable clinical conditions (heart rate of 95 bpm, oxygen saturation of 96%, and a respiratory rate of 23 rpm) in ambient air.
The clinical bedside examination of swallowing (for the screening procedure) was designed to identify the presence or absence of swallowing disorders (abnormal anatomy or physiology).
SURFACE ELECTROMYOGRAPHYSwallowing was noninvasively detected and monitored by surface electromyography (EMGs) (Lynx Tecnologia Eletrônica Ltda, Brazil, 8-channel amplifier, model PA1280, and an A/D converter, model ADS 1200-8-4l; 12 high-precision channels for the acquisition of 16-bit signs with an input range of ± 5 volt configured for an amplification frequency of 1000 Hz and a predetermined recording time of 30 s) in order to detect infrahyoid muscle activity during swallowing via the skin–surface electrode (trace hal double/bipolar, Miotec) on the cervical region. The data from these measurements (Figure 1) are expressed in millivolts (mV) or microvolts (µV).19,20
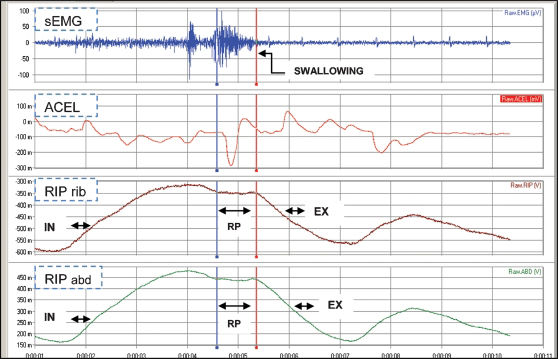
Graphical depiction of the signals that were obtained from surface electromyography (sEMG), accelerometry sensor (ACEL), and inductive respiratory plethysmography (RIP) measurements in patients who swallowed 10 mL of water.- Graphical depiction of the signals that were obtained from surface electromyography (sEMG), accelerometry sensor (ACEL), and inductive respiratory plethysmography (RIP) measurements in patients who swallowed 10 mL of water.
Notes: Respiratory phases: inspiration (IN), expiration (EX), respiratory pause (RP), plethysmography rib cage (RIP rib), and plethysmography abdomen (RIP abd).
The electromyograph was directly connected to a personal PC notebook, a Toshiba Satellite, which was equipped with a dual-core Intel Pentium processor, software, and the signal acquisition program, BioInspector 1.8 (AqDados and AqDAnalysis of Lynx Tecnologia Eletrônica Ltda © 2008).
All of the collected data were archived as AqDados 7 or type-text formats onto the computer's hard disk for the offline analysis of swallowing duration, respiratory cycles, and the respiratory pause that is associated with respiratory movements. The data were collected for the parameters that are associated with the onset and outset of respiratory cycling (minimum, maximum, media, and standard deviation).
SWALLOWING ACCELEROMETRYAn acceleration sensor (Piezo Sensor, 100 Mini sense, Biolink Medical) was also used to register the physiological vibration signals from the neck during the anterior–superior hyolaryngeal movements between the thyroid cartilage and cricoids.21
The amplitude of the sensor input was band-pass-filtered with cut-off frequencies at 0.01–20.00 Hz. The integrated signals from the sEMG and sensors were processed in order to detect the start (onset), maximum peak and return, and the initial position (offset) before swallowing.
INDUCTANCE RESPIRATORY PLETHYsMOGRAPHYInductance respiratory plethysmography was used to non-invasively monitor thoracic and abdominal movements during the process of swallowing. RIP was positioned around the thorax above the line of the nipple and around the abdomen at the umbilical level. (Respitrace plus, Ardsley, NY).22
PROTOCOLThe study participants were comfortably seated, and the protocol was initiated after a period of quiet breathing (1 minute). The study participants were asked to maintain their heads in a neutral position to avoid head shifts during swallowing. Water boluses were placed in the participants’ mouths using a glass (50 mL) according to previously determined bolus volumes that were randomly selected [3, 5, and 10 mL] and taking care to not use the same bolus size twice consecutively. Three sets of three volumes were used during the protocol (the total administered water volume was 65 mL), and all of the participants were blinded to the bolus size. The participants were instructed to normally swallow while trying to be as efficient as possible. The total recording time in this protocol was about 20 minutes.
DATA ANALYSISThe onset of swallowing was identified by the onset of the phasic muscular sEMG activity signal, and the offset was determined from the beginning of the downward hyolaryngeal movement as detected by the piezo sensor. Respiratory movement was analyzed according to the traced direction (inspiration [up] or expiration [down]), which was detected by the RIP and characterized according to the patterns of coordination between swallowing and the phases of the respiratory cycle:
- •
Pattern 1: expiration–expiration (EX–EX)
- •
Pattern 2: expiration–inspiration (EX–IN)
- •
Pattern 3: inspiration – expiration (IN–EX)
- •
Pattern 4: inspiration – inspiration (IN–IN)
Pause respiration was determined by the plateau in the respiratory trace along the abscissa in the positive or negative directions as detected by RIP. For each bolus size, we calculated the duration of swallowing, frequency of swallowing, presence and duration of pause respiration, and coupling in the respiratory cycle.23
RESULTSFigure 2 is a graphical illustration of the signal integration of the respiration–swallowing interactions in preliminary study participants during the action of swallowing water.
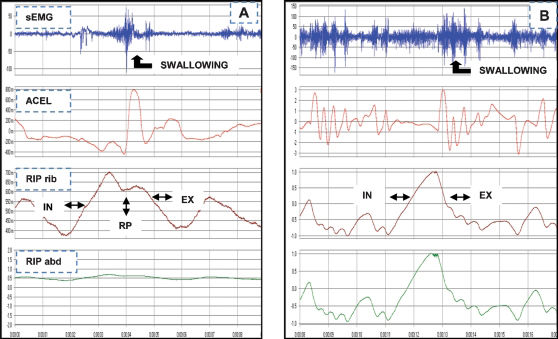
Graphical demonstration of the integrated signals of the surface electromyography (sEMG), accelerometry sensor (ACEL), and inductive respiratory plethysmography (RIP) measurements in healthy volunteers (A) and patients (B) who swallowed 10 mL of water.- Graphical demonstration of the integrated signals of the surface electromyography (sEMG), accelerometry sensor (ACEL), and inductive respiratory plethysmography (RIP) measurements in healthy volunteers (A) and patients (B) who swallowed 10 mL of water.
Notes: Respiratory phases: inspiration (IN), expiration (EX), respiratory pause (RP), and plethysmography rib cage (RIP rib).
The healthy volunteer in this study presented a mean duration of swallowing (15 episodes) of 1.21 s and only one swallowing act per respiratory cycle (Table 1), which was the most frequent combination. The onset and offset of the swallowing in combination with the expiratory phase of the respiratory cycle was associated with the presence of a respiratory pause of swallowing (mean time to pause: 0.62 s).
The patient in this study exhibited 39 swallowing episodes with a mean duration of 1.43 s (Table 1), whereas three to four swallows per respiratory cycle were the most frequent combination. The onset and offset of swallowing were coupled in the phases of expiration–expiration (pattern 1) and inspiration–expiration (pattern 2) in 60% and expiration–inspiration and inspiration-inspiration in 40% of the respiratory cycles (pattern 3 and 4) that were associated with swallowing.
DISCUSSIONThe technology that was developed in this study was capable of simultaneously recording the occurrence of swallowing and respiratory movements, respiratory pause, and coupling with the respiratory cycle during water swallowing in a healthy volunteer and a patient who had been admitted to an intensive care unit. Variations in the stable pattern are clinical indicators for the risk of aspiration due to compromised airway protection mechanisms.
This average duration of respiratory movements during liquid swallowing in a healthy adult and the onset of the pause contained therein have been associated with adequate airway protection during hyolaryngeal movement; however, a change in the coupling of swallowing with the respiratory cycle of inspiration–inspiration (40%) was detected in the patient, suggesting that the patient was at risk for aspiration.
This preliminary study demonstrates that it is possible to visualize and measure respiratory activity as well as its interaction with swallowing at the bedside in an intensive care unit. The technology that was used in this protocol was efficient, and the following results were observed:
- •
The method was non-invasive and portable, which permitted bedside recording of the respiration–swallowing interactions of a patient who had been submitted to endotracheal intubation after extubation.
- •
The method was objective, rapid (approximate 20 minutes), and permitted bedside measurements (qualitative and quantitative) of respiration–swallowing patterns as well as the evaluation of the effect of therapeutic strategies in the rehabilitation of swallowing.
- •
It was a readily accessible method for evaluation that can be used in different populations, such as infants, healthy elderly patients, or those at risk for aspiration.
Our initial results suggest that the methodology and technology discussed here permit a non-invasive detection of bedside respiration signals and swallowing patterns. Further studies are needed to understand the clinical implications of the respiration and swallowing interaction.