Coronary arteries normally arise from the sinuses of Valsalva on the ascending aorta. The incidence of anomalous origin of the left coronary artery from the trunk of the pulmonary artery is about 1 in 300,000 live births.1
The clinical course of patients with this anomaly, which includes heart failure early in life, depends on either the development of coronary collaterals after birth2 or invasive correction.3–4 Here, we report a case of a five-year-old female with exertional dyspnea and changes in her electrocardiographic examination who was referred for magnetic resonance imaging (MRI).
Case PresentationThe patient was a five-year-old Caucasian, female, born at term with no history of complications during labor. In the fourth month of life, she developed prolonged crying during feedings. A constitutional growth delay was discovered at her first consultation with her pediatrician. In her seventh month of life, the child exhibited mild dyspnea and cyanosis during feeding. The patient presented clinically with delayed physical development and was always below the growth curve at her regular consultations. Nevertheless, there were no suspected abnormalities based on her examinations until the age of five. Symptoms of heart failure were detected when the patient began school; she suffered from constant fatigue, and her dyspnea was increased and occurred earlier than her colleagues during physical exercise. The child underwent a clinical examination by a cardiologist, and the results of the electrocardiogram (EKG) suggested a change in heart vascularization.
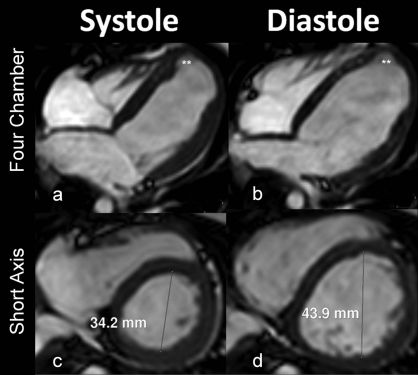
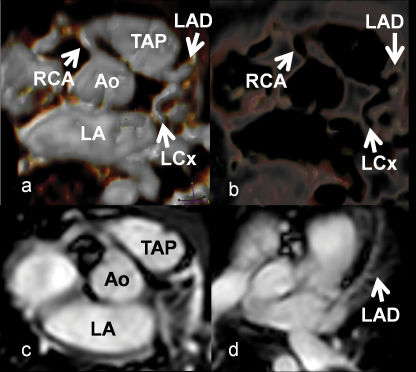
The patient was referred for an MRI using a 1.5-Tesla Achieva system (Philips Medical Systems, Best, The Netherlands) with a five-element cardiac phase-array coil. The study was performed under anesthesia with gadolinium contrast (0.2 mmol/kg). The multiplanar images (Figure 1) obtained from the cardiac MRI synchronized to the ECG during full expiration and the magnetic resonance angiography of the coronary arteries (Figure 2) were analyzed and processed in using an Extended MR WorkSpace 5.2 workstation (Philips Healthcare, Best, The Netherlands).
Magnetic resonance angiography of the coronary arteries with 3D reconstructions showing the ostia of the coronary arteries. Note that the left coronary artery has its origin in the pulmonary trunk (TAP – trunk of pulmonary artery, Ao - ascending aorta, LA - left atrium, Cx - circumflex coronary artery, DA - left anterior descending coronary artery, CD - right coronary artery).
The rare anomaly of the origin of the left coronary artery1 from the trunk of the pulmonary artery (ALCAPA - Anomalous Left Coronary Artery from Pulmonary Artery) is often referred to as Bland-White-Garland syndrome. The estimated incidence of Bland-White-Garland syndrome is 1/300,000 live births and represents 0.5% of congenital heart disease cases. During fetal life, this anomaly is tolerated because there is no difference in blood pressure between systemic and pulmonary circulation, nor is there a difference in the oxygen gradient between the aorta and the pulmonary artery6. The presence of symptoms depends on the type of collateral circulation between the right and left coronary arteries. Development of these collaterals may be crucial for the onset of myocardial ischemia.
The anomaly can be classified according to collateral pattern. When collaterals are present it is called the “adult type”, and the “infantile type” occurs when there are no collaterals.7 Patients usually show signs and symptoms of congestive heart failure due to ischemic heart disease and myocardial infarcted segments.1,8,9
The infantile form has a potential mortality of about 80–90%, even with medical treatment, and exhibits clinical symptoms of myocardial ischemia, such as sweating, irritability and fatigue.10 The onset of symptoms often occurs around the eighth week of life. An important differential diagnosis in adults is dilated cardiomyopathy.5,10
In the adult form, patients usually have significant collateral circulation between the right and left coronary arteries; however, the amount of blood perfusion through the collaterals is not adequate, especially in the subendocardial region. Therefore, the disease manifests as chronic ischemia, which can result in a malignant ventricular arrhythmia and a risk of sudden death in 80–90% of cases. Patients with this form of the anomaly can also be asymptomatic.2
Coronary artery angiography can characterize changes in the origin, course and morphology of the coronary tree. It is invasive and has a low, but present, risk of complications. MRI is a noninvasive method that does not require radiation and can demonstrate the origin of the coronary arteries and assess the degree of valvular insufficiency, ventricular function, regional contractility and myocardial viability, all of which are important considerations during the preoperative evaluation and postoperative follow up.7,9
Surgery is the proposed treatment for both forms of ALCAPA.3,4 The Takeuchi procedure is a technique used to correct the infantile form of this syndrome, which consists of creating a communication between the aorta and the left coronary ostium, through the pulmonary artery, using tubular material (graft).7 Usually, this technique is performed when direct implantation of the anomalous artery into the aorta is difficult due to unfavorable conditions. In the adult form, ligation of the origin of the coronary artery at the pulmonary artery is performed in a combined manner so that flow is either restored or persists through a connection with either the internal thoracic artery or a saphenous vein graft from the ascending aorta.11
Final considerations: The patient described in this case report underwent surgical ligation of the origin of the left coronary artery, and flow was restored by a graft to the left internal mammary artery. The patient had a good recovery in the early postoperative period and was discharged with her cardiac function under control.
All authors were involved in the conception and design of this manuscript, the drafting the manuscript, critical revisions of the manuscript for intellectual content, and final approval. Marcelo Souto Nacif was involved in the data acquisition.