The physiopathology of insulin resistance in women with polycystic ovary syndrome (PCOS) is related to a disturbance in the function of the insulin receptor. In fact, the post-receptor defect associated with PCOS may be a critical factor that interferes with the recruitment of proteins for intracellular glucose transport. The conceivable end result is a compensatory increase in insulin (1). One possible option for correcting this insulin resistance is the use of drugs (such as metformin and glitazones) that may increase glucose intake in the tissue (1,2). However, there are studies showing that a few patients interrupted their metformin treatment due to a high incidence of gastrointestinal side effects, such as nausea or vomiting (2).
Rosiglitazone binds to the peroxisome proliferator-activated receptor, which regulates the transcription of many genes, including the glucose transporter, and decreases insulin resistance (4); however, this drug may increase the risk of cardiovascular diseases, such as myocardial infarction. These effects are not reported in patients who have insulin resistance without diabetes (5). It is important to emphasize that endothelial damage is more pronounced in diabetic patients than in non-diabetic ones (6).
Approximately 1% of IGF-1 circulates freely in the plasma; the remainder is transported by binding proteins. The efficacy of muscle IGF-1 depends on the expression and availability of a family of six types of binding proteins. In humans, the most important of these proteins is IGFBP-3 (>80%), which is responsible for the maintenance of the circulating IGF-1 levels, along with the ALS glycoprotein, which has great affinity for IGF-1 and -2 (7). The increase in insulin may affect IGF-1 actions. In fact, insulin decreases the production of IGFBP-3 in the liver. Therefore, the free levels of IGF-1 are elevated and may affect ovarian function and increase androgen production (8). The aim of this study was to evaluate the actions of rosiglitazone on IGF-1 and IGFBP-3 in women with PCOS.
MATERIALS AND METHODSThis study was conducted at the General Gynecology Outpatient Clinic, University Hospital, Federal University of Paraíba, from 2007-2008. The protocol was approved by the Institutional Ethics and Research Committee (≠ 405/2004) and Clinical Trials (PFU-IRB#405/2004). The patients were randomly assigned to two groups: rosiglitazone and placebo. All patients signed informed consent statements.
The PCOS diagnosis was based on the AES-2006 Criteria (3). The exclusion criteria included the following: the use of hormonal contraceptives; other causes of anovulation (i.e., Cushing's syndrome, hyperprolactinemia, adrenal enzyme deficiency, thyroid disorders, and adrenal or ovarian tumors); high serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels (e.g., a threefold increase in the normal level); serum levels of total cholesterol over 300 mg/dL; or triglycerides higher than 400 mg/dL. Also excluded were users of drugs, hormones or substances that might interfere with carbohydrate metabolism up to three months prior to the study or patients who had recently started intensive physical exercise (i.e., aerobics).
Selection of patientsThe study procedures included the performance of anamnesis and a general physical and gynecological examination; the collection of anthropometric data (including measurements of weight [kg], height [m], abdominal circumference [AC, cm], hip circumference [HC, cm]), and blood pressure with a sphygmomanometer [mmHg]; a calculation of body mass index [BMI, weight/height2] and waist-hip ratio [WHR, AC/HP]; and a classification of hirsutism according to Ferriman & Gallwey (9). A patient was deemed hirsute if her index was over 8. All patients were advised to use a non-hormonal contraceptive method.
Measurements were made of the following factors: follicle-stimulating hormone, luteinizing hormone, 17 beta-estradiol, free and total testosterone, thyroid-stimulating hormone, free thyroxine (T4), 17 hydroxyprogesterone (17OHP), dehydroepiandrosterone sulfate (DHEA-S), GH, cortisol, androstenedione, prolactin, and serum levels of IGF-1, IGFBP-3, and SHBG. Blood was collected to determine the lipid profiles and the glucose and insulin levels for both treatment groups. The participants received 75 g of saccharose, followed by collection of another blood sample 2 hours later (10). The following tests were also conducted: creatinine, hemogram, and beta-human chorionic gonadotropin assays. These measures were made before and after treatment. Additionally, blood samples were collected for FSH and LH after the third day of the menstrual cycle from cycling patients and on any day from patients with amenorrhea.
After interviewing patients to collect their clinical histories and subject them to general physical and gynecological examinations, 60 women were selected to participate. Each patient received capsules labeled ZX or XZ and information about the meaning of these labels. Each capsule taken by the ZX group contained 4 mg of rosiglitazone maleate (Avandia®, GlaxoSmithKline), and each capsule taken by the XZ group contained starch (e.g., placebo, an inactive substance). Participants were instructed to take the medication orally, twice a day, at 12-hour intervals, during three months.
The presence of insulin resistance was determined by the HOMA IR (11) and QUICK (11,12). The HOMA-IR was used to evaluate insulin sensitivity, and values >2.7 were considered to be indicative of insulin resistance (13). Only 33 women completed the 12 weeks of treatment and were included in the statistical analyses (Figure 1). We evaluated the diary card for the days of menstruation, compliance with medication use (e.g., the number of consumed capsules), and the side effects. We excluded the patients who did not take at least 90% of their medication (n = 4) or who had become pregnant (n = 4).
Statistical AnalysisThe following tests were used for analysis Wilcoxon, Mann-Whitney, Pearson correlations and Chi-square. The data from the variables were organized using Excel spreadsheets and were then converted to files using the statistical program Statistical package for the Social Sciences (SPSS, DMSS, www.spss.com.br), version 13.0. p-values smaller than 0.05 were regarded as statistically significant.
RESULTSThe anthropometric data and the summaries of the clinical data on menstrual patterns, cutaneous repercussions of hyperandrogenism (i.e., acne), and acanthosis nigricans (e.g., insulin resistance) are shown in Table 1. The hormonal data and levels of serum SHBG, glucose, insulin, type 1 insulin growth factor (IGF-1), and its binding protein (IGFBP-3) are shown in Table 2.
Clinical data for the women with PCOS in the placebo and rosiglitazone groups.
| Variable | Placebo (n = 17) | Rosiglitazone (n = 16) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | After treatment | p-value | Baseline | After treatment | p-value | ||
| Menstrual Pattern | Oligomenorrhea (%) | 58.8 | 64.7 | 0.564 | 50.0 | 37.5 | 0.026 |
| Amenorrhea (%) | 41.2 | 35.3 | 50.0 | 0.00∗ | |||
| Regular (%) | 0.00 | 0.000 | 0.000 | 0.625 | |||
| Age (years) | 26.29±5.31 | 26.29±5.31 | 1.000 | 24.56±4.33 | 24.56±4.33- | 1.000 | |
| Weight (kg) | 72.65±15.82 | 72.45±16.63 | 0.987 | 68.20±14.39 | 68.15±15.29 | 0.983 | |
| BMI (kg/m2) | 30.09±6.51 | 30.19±7.42 | 0.976 | 27.89±6.10 | 27.79±7.20 | 0.972 | |
| WHR | 0.87±0.12 | 0.86±0.22 | 0.950 | 0.88±0.86 | 0.87±0.75 | 0.970 | |
| Height (m) | 1.55±0.05 | 1.55±0.05 | 1.000 | 1.56±0.06 | 1.56±0.06 | 1.000 | |
| Ferriman-Gallwey index | 9.76±4.92 | - | 8.81±3.76 | - | |||
| Acne (%) | 35.3 | 29.4 | 0.317 | 37.5 | 6.3∗ | 0.025 | |
| AN (%) | 47.1 | 47.1 | 1.000 | 48.3 | 12.5∗ | 0.045 | |
The data are summarized as means ± standard deviations in both groups (n) = Number of women. Acronyms: BMI = body mass index, WHR = waist hip ratio, AN = acanthosis nigricans. Hirsutism reported by the patients. The results for menstrual cycle pattern, acne, hirsutism, and AN were analyzed using the chi-square test. The Wilcoxon test was applied for comparing the results between placebo and rosiglitazone. ∗p< 0.05 compared to the placebo group.
Hormone values, SHBG values, and glycemia, insulin, type 1 insulin-like growth factor (IGF-1), and type 3 insulin-like growth factor-binding protein (IGFBP-3) levels of the women with PCOS in both the placebo and rosiglitazone groups (means ± standard deviations).
| Variable | Placebo(n = 17) | Rosiglitazone(n = 16) | ||||
|---|---|---|---|---|---|---|
| Beforetreatment | Aftertreatment | p-value | Beforetreatment | Aftertreatment | p-value | |
| FSH (mIU/mL) | 5.90±1.78 | 4.75±1.55 | 0.023 | 4.75±1.15 | 4.23±1.58 | 0.277 |
| LH (mIU/mL) | 15.71±16.02 | 10.95±5.32 | 0.619 | 10.88±5.37 | 8.72±6.78 | 0.163 |
| E2 (nmol/mL) | 73.77±81.38 | 75.03±41.38 | 0.368 | 56.82±21.49 | 81.94±72.02 | 0.605 |
| 17OHP (mg/mL) | 1.71±0.89 | 1.99±00.97 | 0.408 | 1.79±0.90 | 1.45±0.56 | 0.352 |
| DHEA-S (mg/mL) | 225.00±83.60 | 215.68±64.43 | 0.586 | 209.09±60.18 | 179.54±54.39∗ | 0.026 |
| SHBG (nmol/mL) | 32.82±15.22 | 30.94±12.04 | 0.836 | 32.29±11.61 | 45.18±15.56∗ | 0.030 |
| T total (mg/dL) | 0.78±0.26 | 0.73±0.28 | 0.266 | 0.72±0.27 | 0.60±0.27 | 0.570 |
| A (ng/mL) | 3.86±1.51 | 4.00±1.39 | 0.507 | 3.11±1.18 | 2.15±0.68∗ | 0.002 |
| T free (ng/dL) | 2.91±2.00 | 2.78±1.69 | 0.925 | 2.47±0.99 | 1.37±0.57∗ | 0.006 |
| Glucose(mg/dL) | 80.91±14.02 | 84.88±5.64 | 0.179 | 87.94±7.40 | 78.43±13.85 | 0.488 |
| Insulin(µIU/mL) | 13.20±9.07 | 14.81±8.99 | 0.309 | 13.38±7.36 | 12.50±9.46 | 0.379 |
| HOMA IR | 3.30±1.92 | 2.82±2.04 | 0.163 | 2.96±1.81 | 2.22±1.56 | 0.251 |
| QUICK | 0.33±0.27 | 0.34±0.40 | 0.065 | 0.33±0.27 | 0.35±0.33 | 0.379 |
| G 2h | 117.76±31.90 | 95.00±19.94 | 0.001 | 114.62±35.59 | 104.31±34.4 | 0.020 |
| I 2h | 103.50±64.38 | 92.69±70.31 | 0.535 | 112.98±88.74 | 64.04±64.23∗ | 0.001 |
| G 2h/I 2h | 1.46±0.65 | 1.58±0.69 | 0.492 | 1.37±0.58 | 2.30±1.21∗ | 0.002 |
| IGF-1(ng/mL) | 186.24±66.45 | 218.88±70.31 | 0.005 | 196.12±68.25 | 176.94±53.31∗ | 0.028 |
| IGFBP-3(ng/mL) | 5.43±0.93 | 5.01±1.16 | 0.201 | 4.86±0.82 | 5.34±0.70 | 0.041 |
17OHP = 17 hydroxyprogesterone; T = testosterone; A = androstenedione. The Wilcoxon test was used for comparing the measurements made in each group (placebo or rosiglitazone) before and after treatment. HOMA-IR and QUICK are mathematical equations for evaluating insulin resistance. G2h – glycemia after 2 h of glycemic overload with 75 g of saccharose; I 2h – insulin after 2 h of glycemic overload with 75 g of saccharose. The Wilcoxon test was applied for comparing the results between placebo and rosiglitazone. ∗p<0.05 compared to the placebo group after treatment using the Mann-Whitney test.
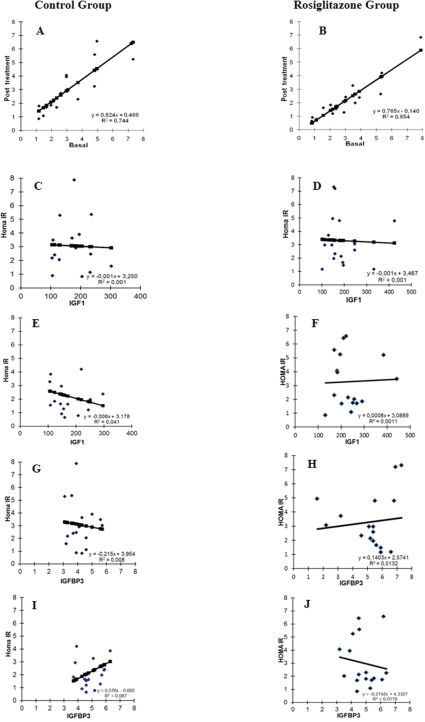
Figure 2 shows the linear regression data. Graphs A and B show that the coefficient of determination (R2) of the rosiglitazone group was greater than that of the control group. The values of the two regression equation slopes decreased from 0.824 to 0.765, indicating that patients in the group treated with rosiglitazone had a significant decrease in their HOMA-IR values. These data show that compared to the control group, rosiglitazone significantly decreased the HOMA-IR values. The effects of rosiglitazone on IGF-1 levels and the HOMA-IR are represented in Figure 2 (C-F). Results indicate that compared to the placebo group, the IGF-1 levels decrease in parallel with the values of HOMA-IR following rosiglitazone treatment (Fig 2, E-F, p<0.01). We did not identify any differences in the correlations of HOMA-IR and IGF-1 between the two groups before the treatment (C-D). The opposite effect of rosiglitazone was observed in the comparisons between the HOMA-IR values and the IGFPB-3 levels. Low HOMA-IR values were correlated with high levels of IGFPB-3 after treatment (Fig 2, G-H, p<0.01). We also did not observe any differences in the correlations of the HOMA-IR values and the IGFPB-3 levels before treatment across the two groups (Fig. 2 I-J).
Pearson's correlation between insulin resistance and IGF-1 and IGFPB-3. A – Correlation of HOMA-IR values before vs. after treatment in the control group; B - Correlation of HOMA-IR values before vs. after treatment in the rosiglitazone group; C – Correlation of HOMA-IR values with IGF-1 levels before treatment in the control group; D - Correlation of HOMA-IR and IGF-1 levels before treatment in the rosiglitazone group; E - Correlation of HOMA-IR values with IGF-1 levels after treatment in the control group; F - Correlation of HOMA-IR values with IGF-1 levels after treatment in the rosiglitazone group; G - Correlation of HOMA-IR values with IGFPB-3 levels before treatment in the control group; H - Correlation of HOMA-IR values with IGFPB-3 levels before treatment in the rosiglitazone group; I - Correlation of HOMA-IR values with IGFPB-3 levels after treatment in the control group; J - Correlation of HOMA-IR values with IGFPB-3 levels after treatment in the rosiglitazone group.
Various drugs can be used in the treatment of PCOS, including oral contraceptives, anti-androgens, anti-estrogens and, more recently, insulin-sensitizing agents (i.e., biguanides and thiazolidinediones) (14). The latter agents have been used to reduce hyperinsulinemia, including its negative impact on ovarian function and long-term cardiovascular consequences (15). Insulin-sensitizing agents are replacing the well-established and frequently used oral contraceptives, which aggravate the insulin resistance and glucose intolerance induced by increasing the risk of developing type 2 diabetes, elevating triglyceride levels, and raising the cardiovascular risks, due to the oral contraceptives actions on coagulability and vascular reactivity (16). Some authors have suggested that such a change in carbohydrate metabolism occurs in 70% of women (17). Although not all of our patients exhibited insulin resistance, we found a correlation between the HOMA-IR value and IGF-1 or IGFPB-3 levels.
Although the exact pathogenesis of PCOS still remains unknown, the insulin resistance and hyperinsulinemia appear to play key pathogenetic roles in the ovarian overproduction of androgens (12,13). The SHBG levels increased with therapy, further reducing the bioavailability of circulating androgens. These changes were most likely caused by an improvement in insulin sensitivity, resulting in the amelioration of hyperinsulinemia and thus the reduction of ovarian androgen production (18,19). In fact, our study showed that rosiglitazone indeed influenced hormone profiles, with reductions observed in both free and total testosterone levels in women with PCOS.
The decrease in peripheral insulin resistance in PCOS resulting from the employment of metformin and rosiglitazone may lead to diminished hyperinsulinemia and may interrupt the triggering and perpetuating mechanisms of hyperandrogenism in PCOS patients (20,21). In our study, we identified a correlation between the amelioration of insulin resistance and decreased IFG-1 and IFGBP-3 levels. We also observed a decrease in the free testosterone fraction and an increase in SHBG. Hirsutism was not evaluated in this study because the hair follicle cycle ranges from six months to two years (6,22), thus requiring a more prolonged treatment to observe any changes than was possible in the time frame of this study. However, acne, which is also dependent on hyperandrogenism, improved in our patients (23).
There was a post-treatment reduction in the IGF-1 levels in the women who took rosiglitazone. This result is controversial in the literature. In patients with acromegaly (a disease that raises GH and IGF-1 levels), rosiglitazone did not reduce IGF-1 or its binding proteins (24,25). There was, however, a decrease in these factors in patients with both acromegaly and diabetes mellitus (26,27). In fact, insulin may diminish IGF-1 and its binding proteins at the hepatic level, thus limiting the effects of IGF-1 and, therefore, ovarian stimulation (28).
Our results demonstrated that rosiglitazone might improve hyperandrogenism and insulin resistance in PCOS women. These beneficial effects were observed with the three-month treatment. It is also relevant that the menstrual patterns normalized during this interval of observation.
AUTHOR CONTRIBUTIONSBatista JG conducted the study, analyzed and interpreted the data, and drafted the paper. Soares-Jr JM analyzed the data, revised the draft of manuscript, and approved the version to be published. Maganhin CC analyzed the data and revised the draft. Baracat EC helped to conceive the study, analyze the data, and critically revise the manuscript for important intellectual content. Simões RS revised the final version of the paper. Tomaz G contributed to the conception and design, and to the acquisition, analysis, and interpretation of the data.
This study was supported by the Federal University of São Paulo, the Federal University of Paraiba, and CAPES (financial support).
No potential conflict of interest was reported.