To evaluate the outcomes and diagnostic performance of ultrasonography after a Breast Imaging Reporting and Data System (Bi-RADS) category 0 mammogram.
MATERIAL AND METHODS:This retrospective study reviewed 4,384 consecutive patients who underwent a screening mammography from January 2005 to July 2006; 391 of the 4,384 exams were classified as Bi-RADS category 0. After exclusions, 241 patients received subsequent sonogram. Ultrasonography was considered diagnostic when the Bi-RADS category was changed to 2, 4, or 5, and it was considered indeterminate (Bi-RADS 3) when the results indicated that the patients should return for a mammographic follow-up. The outcomes of these patients were assessed to evaluate the diagnostic performance of ultrasonography.
RESULTS:The mean age of the patients was 53.3 years (ranging from 35 to 81). Of the 241 patients, ultrasonography was considered diagnostic in 146 (60.6%) patients and indeterminate in 95 (39.4%) patients. In the diagnostic group, 111 out of 146 patients (70.2%) had a sonogram result of Bi-RADS category 2 after a 2-year follow-up without evidence of malignancy. Furthermore, 35 out of 146 patients (29.8%) had a suspicious sonogram with a result of Bi-RADS category 4. After a tissue sampling procedure, 10 patients were confirmed to have breast cancer, and 25 had benign histopathological features without any evidence of malignancy after a 2-year follow-up. The sensitivity of ultrasonography was 100%, specificity was 89.1%, and overall accuracy was 89.6%.
CONCLUSIONS:Based on the degree of resolution and its diagnostic performance, ultrasonography was determined to be an excellent method for the subsequent evaluation of Bi-RADS 0 mammograms.
The imaging approach for the evaluation of breast lesions was standardized with the introduction of the Breast Imaging Reporting Data System (Bi-RADS), which was created by the American College of Radiology (ACR) in 1992.1 This systematization is a data control system that provides a lexicon for describing lesions, establishes levels of suspicion for breast cancer, and indicates the required subsequent steps for the evaluation and treatment of breast cancer. Six categories exist for breast lesions in Bi-RADS. An extra category, Bi-RADS 0, is reserved for situations in which an additional method may improve lesion characterization. Ultrasonography (US) plays an important role in the detection of breast lesions that are either palpable or only detected by imaging. Over the last 30 years, a consensus has been reached regarding the indications for breast sonography.2–4 US is the current choice for the evaluation of dense breasts in young patients, the differentiation of cystic and solid lesions, and for guidance procedures. The capability of US as a screening method for breast cancer remains controversial.5–10 Nonetheless, US has traditionally been the preferred adjunctive method when further evaluation is required after mammography.11–12 However, the impact of sonography after a Bi-RADS 0 mammogram has not been thoroughly investigated in the literature. Although some authors have addressed the value of US in Bi-RADS categories 3 to 5,13–14 no studies have assessed US performance and its resolution after a Bi-RADS category 0 mammography.
Therefore, we conducted this retrospective study to investigate the performance of US as a secondary diagnostic tool and to assess the outcome of mammograms that were initially classified as Bi-RADS category 0.
MATERIALS AND METHODSStudy DesignThis retrospective study was approved by the Institutional Review Board of our institution. Informed consent was not required. All consecutive screening mammography data collected between January 2005 and July 2006 were reviewed in the institutional database. Our institution performs both screening and diagnostic mammographies. Mammograms that were classified as Bi-RADS category 0 were extracted for further evaluation. For each patient, we recorded the method used, the results obtained, and the approach adopted after the additional imaging. Medical records were reviewed to determine the final outcome of the patients. The inclusion criteria for this study were as follows: a) patients with Bi-RADS category 0 mammograms that were further evaluated by US and b) patients without tissue sampling who had histopathological diagnoses and/or a two-year follow-up. A two-year follow-up interval was chosen because it is the period of time normally used in the literature to define stable lesions.1,9 Exclusion criteria were as follows: a) patients with palpable lesions, b) patients whose mammograms were classified as Bi-RADS category 0 (i.e., further evaluation) because a previous examination was not available, c) patients who had a previous diagnosis of breast cancer, and d) patients who were participating in follow-up studies for lesions that were proven benign, whether with or without breast manipulation.
Imaging techniquesAll mammograms were performed using a DMR Mammography System from GE Medical Systems, Milwaukee, USA. Our protocol consisted of routine craniocaudal and oblique mediolateral views for both breasts. Additional tests, such as magnification, compression, axilar, and other views, were performed if necessary. All ultrasound exams were performed with real-time, dynamic equipment (Acuson Aspen, Acuson-Siemens, Mountain View, California, USA), which had a high-resolution, phased-array transducer and a frequency that ranged from 7.0 to 12.0 MHz. Color and Power Doppler were available in all equipment.
Imaging InterpretationMammograms were evaluated and reported by 2 of the authors of this study (J.E. and V.F.M.) who had 13 and 12 years of experience reading mammograms, respectively. For all patients, the mammographic findings were described using the Bi-RADS lexicon. At the end of the experiment, a final Bi-RADS category was reported. In addition to the Bi-RADS category assigned, breast parenchymal density was evaluated according to the Bi-RADS systematization. It was categorized as follows: American College of Radiology density 1 (ACR D1) was categorized as almost entirely fat (less than 25% fibroglandular tissue); ACR D2 was categorized as scattered fibroglandular densities (approximately 25% to 50% fibroglandular); ACR D3 was categorized as heterogeneously dense (approximately 51% to 75% fibroglandular); and ACR D4 was categorized as dense (more than 75% fibroglandular).
Mammograms were classified as Bi-RADS category 0 when the following criteria were met: 1) previous exams were not available for comparison; 2) round or oval lesions, which had either circumscribed or partially obscured margins, were present; 3) focal asymmetry was seen on two orthogonal incidences and persistent after additional views.
All US examinations were performed by third-year residents, but as an institutional rule, patients were re-scanned by one of our faculty who had experience in breast US ranging from 10 to 25 years and who were responsible for the final report. The US examination was considered diagnostic if the Bi-RADS category changed to 2, 4 or 5 based on the definitions suggested by Kubiak et al.15 If any cases needed a follow-up mammography (e.g., due to an asymmetry without corresponding findings in the ultrasound), then the US examination was considered indeterminate, and the mammogram category was changed to Bi-RADS 3. The ultrasonographic criteria for probably benign solid lesions were as follows: a) oval or round shape; b) circumscribed margins; c) isoechoic, hyperechoic, or hypoechoic echo patterns; d) parallel orientation (i.e., “wider than taller”); and e) enhancement or no posterior acoustic features. The lesions that met these criteria were classified as Bi-RADS category 3, and only typical intramammary lymph nodes were classified as Bi-RADS category 2. Lesions were classified as suspicious when one of these criteria was absent.
When indicated, tissue samples were obtained using fine needle aspiration, using core biopsy or after open excision by wire location according to the decision from the Mastology Division. The samples were analyzed by two experienced pathologists who were dedicated to the study of breast diseases and had at least six years of experience in this field.
Statistical AnalysisStatistical analysis included descriptive data and an assessment of the degree of US resolution. The overall performance of US was evaluated according to the pattern of breast composition (D1 to D4, as per the Bi-RADS classification), and according to the morphology of the lesion, it was described on mammography using the Bi-RADS lexicon. Statistical significance and confidence intervals were calculated using computerized statistical software (SPSS, version 12; SPSS, Chicago, Ill., USA). A p-value of less than 0.05 indicated statistical significance. The diagnostic effectiveness of US was evaluated using a confidence interval of 95%.
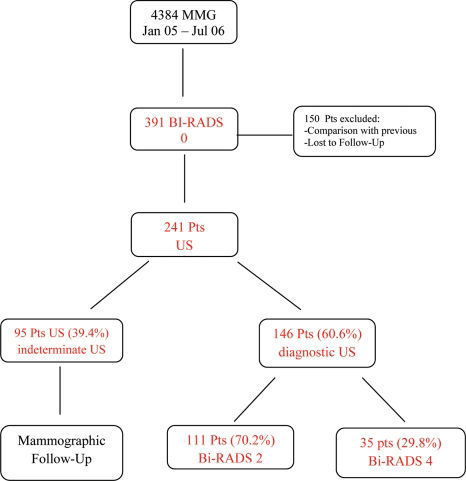
RESULTSFrom the initial 4,384 patients who had a mammography, 391 were classified as Bi-RADS category 0. The mean age of the patients was 53.3 years (ranging from 35 to 81). After applying the inclusion and exclusion criteria, 241 patients had US, when adjuvant method was indicated.
All of the lesions or suspected findings in mammograms in our study were nonpalpable, and the distribution according to the Bi-RADS lexicon was as follows: 62 out of 241 (25.7%) were round or oval circumscribed lesions; 81 out of 241 (33.6%) were round lesions but with obscured margins; 21 out of 241 (8.8%) were mass-like asymmetries; 38 out of 241 (15.7%) were focal asymmetries; 10 out of 257 (3.9%) cases had multiple round, circumscribed lesions; and the remaining 29 cases had mixed findings (11.3%). Among the 391 Bi-RADS category 0 mammograms, 302 (77.2%) required additional views before a final classification was made.
After US, the exams of 95 out of 241 (39.40%) patients were considered indeterminate, with Bi-RADS category 3. In 146 cases (60.6%), US was considered diagnostic. In the diagnostic group, US led to changing the Bi-RADS category to benign (category 2) in 70.2% of cases (111/146), and for the remaining 35 cases (29.8%), US led to a reclassification of the level to category 4 (suspicious). None of the US outcomes were classified as Bi-RADS category 5. For the 111 patients who had Bi-RADS reassigned to category 2, US found cysts in 72 patients (64.9 %), ductal ectasia in 13 cases (11.7%), ultrasonographic benign-appearing masses in 21 cases (18.9%), and other benign features, such as linear scars, in 5 (4.5%) cases. The study results are summarized in the flowchart (Table 1).
Regarding the 35 patients with Bi-RADS category 4, which was determined by ultrasonography, 25 out of 35 (71.4%) patients had benign findings after the tissue was sampled. However, cancer was found in 10 patients (28.6%) from this group: 5 with Invasive Ductal Carcinoma (Figure 1 -A,B,C), 2 with Ductal Carcinoma in situ, 1 with Invasive Lobular Carcinoma (Figure 2-A,B,C), 1 with a malignant Phyllodes tumor, and 1 with an undifferentiated carcinoma according to immunohistochemistry that was probably associated with the uterine cervix. Mass lesions were predominant in our study. Round or oval lesions, either with circumscribed or indistinct margins, represented 62.5% of the total lesions. Over the follow-up period of at least two years, no lesions that were defined as benign by US were revealed to be malignant.
A-Oblique view of both breasts. A focal asymmetry is seen in the left upper quadrant (arrow). B. Magnified craniocaudal view showed an irregular mass lesion and indistinct margins (arrow). C. US revealed an irregular, hypoechoic mass with indistinct margins (arrow). Core biopsy revealed a Ductal Invasive Carcinoma.
A-B - Oblique views of both breasts and a magnification of the central region of the right breast. A subtle round asymmetry is seen in the right breast (arrow in B), which was found to be a cyst (not shown). C. Additionally, US demonstrated an irregular mass, which was revealed to be Invasive Lobular Carcinoma by histology.
The fat composition of the breasts was as follows: 26 with grade 1 composition (10.7%); 109 with grade 2 (45.2%); 75 with grade 3 (30.7%); and 32 with grade 4 (13.3%). Regarding breast density, 6 malignant lesions were found in the ACR-D2 group, 2 in the ACR-D3 group, and 2 in the ACR-D4 group. As mentioned previously, the overall index of resolution for US was 60.6% for patients that were reclassified for Bi-RADS categories 2 and 4. When the diagnostic performance of US was analyzed according to breast parenchymal density, no significant difference was observed between fatty (grades D1 and D2) and dense breasts (grades D3 and D4) using the Chi-Square test, with 101 out of 135 cases (74.8%) for ACR D1 + D2 and 88 out of 107 (82.2%) for D3 + D4 (p = 0.22), respectively. Furthermore, no significant differences were observed for the distribution of cancer lesions (Table 2); 6 out of 135 (4.4%) patients had fatty breasts, and 4 out of 107 (3.7%) patients had dense breasts (p = 0.44).
Diagnostic performance of US and the identification of breast cancers based on breast parenchymal density.
| Breast Parenchymal Density | Grade D1+D2 Fatty Breasts | Grade D3+D4 Dense Breasts) | |
|---|---|---|---|
| Diagnostic US | 101/135 (74.8%) | 88/107 (82.2%) | p = 0.22 |
| Breast Cancers found | 6/135 (4.4%) | 4/107(3.7%) | p = 0.44 |
The overall diagnostic performance of US was calculated assuming that all of the cases that were moved to Bi-RADS category 2 or 3 were followed for a minimum period of two years and thus represent true negative cases. The data are shown in Table 3. The overall accuracy was 89.6%; sensitivity was 100%; specificity was 89.1%; positive predictive value (PPV) was 28.6%; and negative predictive value (NPV) was 100%. When the total number of Bi-RADS category 0 cases evaluated by US was considered, the prevalence of cancer lesions was 10 out of 241 (4.1%).
Diagnostic performance of ultrasonography.
| TEST RESULT | Disease + | Disease - | Total |
|---|---|---|---|
| US + | 10 (TP) | 25 (FP) | 35 |
| US - | 0 (FN) | 206 (TN) | 206 |
| Total | 10 | 231 | 241 |
| Parameter | Formula | Value (%) | 95% Confidence Interval |
|---|---|---|---|
| Sensitivity | TP/TP+FN | 100.0 | 65.5 – 100.0 |
| Specificity | TN/TN+FP | 89.1 | 84.2 –92.7 |
| Accuracy | TP+TN/Total | 89.6 | 83.3 – 94.1 |
| PPV | TP/TP+FP | 28.6 | 15.2 – 46.5 |
| NPV | TN/TN+FN | 100.0 | 97.7 – 100.0 |
| Error Rate | FP+FN/Total | 10.4 | 6.7-13.1 |
| Prevalence | TP+FN/Total | 4.1 | 2.1-7.7 |
Abbreviations: US + = Ultrasound positive for cancer; US - = Ultrasound negative for cancer; FN = False negative; FP = False positive; TN = True negative; TP = True positive; PPV = Positive predictive value; NPV = Negative predictive value.
US is widely used as a diagnostic tool in breast lesion management. The accuracy of US in diagnosing solid breast lesions has been extensively evaluated in the literature, and it has been shown to vary from 68% to 96%.18 A reasonable level of concordance exists for most US indications of breast disease, including its use after a mammogram-rated Bi-RADS 0 classification. Despite the extensive literature regarding the role of US in classifying breast lesions, no studies have addressed the performance of US after a mammogram-rated Bi-RADS 0 classification.
Our study indicates that US provides a very efficient alternative to a screening mammogram for obtaining more information about specific breast lesions, which is the rationale for Bi-RADS category 0. Approximately two-thirds (60.6%) of the mammograms in this study were reclassified by US into more precise categories, which either permitted a safe migration to routine screening (Bi-RADS category 2) or triggered a tissue sampling procedure (Bi-RADS category 4). This percentage is in agreement with several studies that have shown a low cancer yield for breast biopsies indicated by a single imaging method or by a combination of them.19–20 When considering all lesions (Bi-RADS categories 2, 3, and 4), the negative predictive value of US was high (100%), whereas the positive predictive value was low (28.6%). This result can be partially explained by the low prevalence of malignant lesions in this subset of patients (10/241 or 4.1%). However, in this subgroup of patients, cancer was not found that was not revealed by sonography within the two-year follow-up period. We did not address the ability of different sonographic criteria to predict malignancy because the incidence of cancer in our sample population was too low for this determination. The sensitivity of US in our study was presumed to be 100%, which can be attributed to multiple factors. First, a selected population screened by mammography was used, and the correct use of the Bi-RADS classification may have generated a sample population that was dominated by a specific type of lesion (i.e., a mass), which is more amenable to ultrasound evaluation. Additionally, we acknowledge that a verification bias (see comment below) might have overestimated the sensitivity.
A significant fraction of our patients (95/241 or 39.4%) was classified as Bi-RADS category 3. We did not move those patients whose exams were re-classified to Bi-RADS category 3 into the diagnostic group because we focused on a immediate resolution, and discharged these cases as being promptly solved by US.
Given the limitations of mammography for dense breasts,9,21–23 such as the fact that density is considered one of the major factors for reduced screening sensitivity in women before the age of 50, one might expect that US would be more helpful in this subgroup. In a previous study by Leconte et al.,14 the sensitivity of US was equivalent for all grades of density; however, the relative risk for detecting nonpalpable cancers was significantly higher in grades 3 and 4. Similarly, in our study, the US sensitivity was identical for all grades of density. However, no difference was observed in the relative risk for detecting nonpalpable cancers, which considered dense breasts to be grades 3 and 4 and fatty-replaced breasts to be grades 1 and 2. This discrepancy is probably related to different study designs. We only evaluated Bi-RADS category 0, but in the aforementioned study, the authors assessed Bi-RADS categories 1, 2, 4, and 5. In our study, the distribution of Bi-RADS category 0 cases, as determined according to breast parenchymal density, did not show any predominance. All breast parenchymal density grades were almost equally represented, and no significant difference was observed in the performance outcome of US in each category. Although routine association of ultrasonography and mammography in women with dense breasts has been advocated by some authors,8,9,24,25 our study did not assess whether this association improves the assessment of dense breasts.
Because our study selected only Bi-RADS category 0 cases, a predominance of round or oval lesions was observed; other shapes were underrepresented in our series due to the lack of lesions that were rated Bi-RADS categories 4 and 5. Microcalcifications were absent from our series because the current mammographic findings were sufficient for the purposes of the classification and approach definitions and because of the known limitations of US for these patients these particular lesions.26,27
We acknowledge that some limitations are present in our study. We used a conventional mammography system instead of digital equipment. Although digital mammography may improve the visualization of lesion details, the actual impact of this advancement on diagnostic accuracy is limited.28,29 A verification bias may also exist because we cannot be sure that no cancer was present in patients who only had a follow-up session. However, a two-year follow-up was used to define stability; this period of time was considered optimal because it has been used reliably in several other studies.1,30–32 Another important issue was that we did not evaluate the interobserver variability because this was a retrospective study, and the mammograms were classified as Bi-RADS category 0 by one of the authors and not by consensus. Strict adherence to the Bi-RADS criteria could decrease interobserver variability. Regarding the US technique, we also acknowledge that this method is strongly operator-dependent, but in a retrospective study, we could not have the same observers performing all of the US exams. Every physician on our staff had at least eight years of experience in US. Therefore, we expect that interobserver variability was minimized but may not have been eliminated.33
In conclusion, US improves lesion characterization after a Bi-RADS 0 mammogram. This method has the potential ability to alter the management of cases in which a biopsy might be recommended, but the risk of carcinoma is estimated to be relatively low. Because of its high sensitivity and ability to detect lesions regardless of breast density, US performance is recommended as the first choice for follow-up evaluations of lesions that are classified as Bi-RADS 0.