To analyze the prevalence and types of prescribing and dispensing errors occurring with high-alert medications and to propose preventive measures to avoid errors with these medications.
INTRODUCTION:The prevalence of adverse events in health care has increased, and medication errors are probably the most common cause of these events. Pediatric patients are known to be a high-risk group and are an important target in medication error prevention.
METHODS:Observers collected data on prescribing and dispensing errors occurring with high-alert medications for pediatric inpatients in a university hospital. In addition to classifying the types of error that occurred, we identified cases of concomitant prescribing and dispensing errors.
RESULTS:One or more prescribing errors, totaling 1,632 errors, were found in 632 (89.6%) of the 705 high-alert medications that were prescribed and dispensed. We also identified at least one dispensing error in each high-alert medication dispensed, totaling 1,707 errors. Among these dispensing errors, 723 (42.4%) content errors occurred concomitantly with the prescribing errors. A subset of dispensing errors may have occurred because of poor prescription quality. The observed concomitancy should be examined carefully because improvements in the prescribing process could potentially prevent these problems.
CONCLUSION:The system of drug prescribing and dispensing at the hospital investigated in this study should be improved by incorporating the best practices of medication safety and preventing medication errors. High-alert medications may be used as triggers for improving the safety of the drug-utilization system.
Medication is the mainstay of health care. However, the risks of drug therapy and the prevalence of adverse effects have increased, most likely due to an increased number of medication errors.1–3
The pediatric population is known to be a high-risk group, and the number of potential adverse drug events is generally higher in pediatric inpatients than in the adult inpatient population.4–6
A systematic review of the literature on incidents in a neonatal intensive care unit revealed that medication errors were the most frequently reported patient safety event.7 A study of five United Kingdom hospitals investigated the incidence and type of prescribing and administration errors in pediatric patients and found a 13.2% prescribing and a 19.1% administration error rate.6
The need to calculate doses according to the age, weight, and body surface area of children increases the possibility of errors compared to adult patients.8,9 Ambiguous, incomplete, or confusing prescriptions may result in incorrectly understanding drug prescriptions, which in turn can lead to problems in drug dispensing and administration.10–13
Researchers, health care professionals, and institutions have proposed action plans and mechanisms to decrease medication errors and increase patient safety. Monitoring a group of drugs classified as high-alert medications (HAMs) by the Institute for Safe Medication Practices (ISMP, United States of America) is one such approach. This measure is important because the consequences for patients may be severe when there are errors in any or all stages of the use process of such drugs.14
With this background in mind, the purpose of this study was to analyze the prevalence and types of prescribing and dispensing errors in a pediatric unit of a Brazilian university hospital that occurred on prescription order forms containing one or more HAMs and to propose preventive measures for these errors.
METHODSA descriptive cross-sectional research study was carried out in the Pharmacy Department of a Brazilian 476-bed university hospital. We focused on prescriptions from the pediatric unit during the 30-day period between October 6th and November 4th 2008. The relevant institutional review board approved the study.
The pediatric unit has 60 beds and provides health care to several types of medium- and high-complexity medical and surgical patients. Some medications, including some HAMs, are dispensed for replenishing the standard inventory of the pediatric unit. Patient-specific drugs are provided individually for each 24-hour period based on copies of each patient's prescription order forms.
A 30-day period was defined for gathering the data. According to a review by the Medical Records Department, this time interval is representative of the care provided by the pediatric unit.
All copies of the patient-specific prescription order forms containing one or more HAMs for pediatric inpatients that were received in the dispensing sector of the pharmacy between 7 a.m. and 9 p.m. within the data-gathering period were included in the study. We used the list of HAMs as defined by the ISMP to select the prescription order forms.15
Prescription orders for parenteral nutrition, chemotherapy and hemodialysis were excluded because these medications are not dispensed by the hospital pharmacy dispensary.
The data were collected by the principal author with assistance from undergraduate pharmacy students. Three training sessions on the use of the data-gathering instruments were provided for all participants.
No actions or comments were made regarding the pharmacy service during the data-gathering period, and the dispensing process remained unaltered to minimize observation bias. The principal author coordinated and supervised the data gathering and completion of the forms.
The investigators were asked to inform the supervising hospital pharmacist on duty about any issues detected during that data gathering that might have caused risk to the patient.
Definitions and classificationPrescription errorsThe HAM prescription errors were classified based on the definitions established by Dean et al.16 and Rosa et al.13 These classifications were adapted for the HAM use processes and standards at the study site. We therefore included as errors any prescription for which the dosage form, concentration, administration route, dose interval, dilution, or infusion rate (where applicable) were not present in the HAM prescription. Prescribing errors were then classified as follows.
Name of the HAM: The name of medication was incomplete.
Dosage form: The dosage form was incorrect, absent, or unclear (leading to doubts about the proper interpretation).
Concentration, Administration route, Dose interval, Dilution: One of these elements of the prescription was incorrect, absent, or unclear (leading to doubts about the interpretation).
Dose and Infusion rate: Either the dose or the infusion rate was higher than indicated, lower than indicated, unclear, or absent according to the guidelines described by Takemoto.17
Denomination of the HAM: Either the chemical name or the trade name was used instead of the generic name.
Legibility: The prescription components were poorly legible or illegible, according to the classification proposed in Rosa et al.13
The dilution and/or infusion rates were evaluated only for the HAMs administrated by infusion.
Dispensing errorsDispensing errors were identified by checking the prescribed drug against the dispensed medication. The dispensing errors were classified into types and subtypes according to the method of Beso et al.18, with adaptations for the medication use processes in place at the study site, which are described below.
Content errorsOur study added dose interval, dilution, and HAM denomination to the variables included in Beso et al.'s18 classification of errors. An error was assigned when a given dosage form, concentration, and/or dose was not in the prescription order but the medication was dispensed nonetheless. We used the following error classifications.
Dosage form: The form dispensed differed from that prescribed or the drug was dispensed without the pharmaceutical form having been specified by the prescriber.
Concentration: The drug was dispensed at a higher or lower dose than that specified in the order, or the drug was prescribed and dispensed without the concentration being specified.
Drug: The dispensed drug was different from the drug prescribed in the order, or the drug was dispensed without being prescribed.
Dose interval: The medication was dispensed without the dose interval having been prescribed.
Dose: The medication was dispensed at a higher or lower dose than ordered, the drug was dispensed without the dose having been specified clearly or the HAM was dispensed without confirming an unusual or unexpected dose.
Dilution: The medication was dispensed at an incorrect dilution or without a prescription for a given dilution.
HAM Nomenclature: The medication was dispensed using a different nomenclature than that prescribed, without informing the prescriber. Nomenclature errors included deviations from how the drug was prescribed (in terms of generic name, brand name, or chemical name).
Quality deviation: The medication dispensed had altered physical-chemical, microbiological, and/or organoleptic characteristics that were recognizable in the dispensing process, such as an overdue or absent expiration date or damage to the original packaging.
Labeling errorsThe presence of labeling errors, such as incorrect patient and/or drug identification, was verified as instructed by the study worksite, as instructed.
Documentation errorsThe name of the person who dispensed the HAM was poorly legible, illegible or absent in the copy of the prescription order form.
Concomitant prescribing and dispensing errorsPrescribing and dispensing errors were analyzed using the definitions outlined above. We considered prescribing and dispensing errors to be concomitant when it was clear that the prescribing error was the cause of, or closely related to, a dispensing error of the same type.
AnalysisThe data were entered into a database created using the Epi Info version 6 software. The descriptive and analytical statistics were obtained from the Epi Info software (version 6, CDC, Atlanta, USA) and R software (2.7.1 Version, R Foundation for Statistical Computing, Vienna, Austria).
Each prescribed HAM could be associated with more than one dispensing error and/or more than one prescribing error.
The association between concomitant prescribing and dispensing errors was determined using Fisher's exact test.
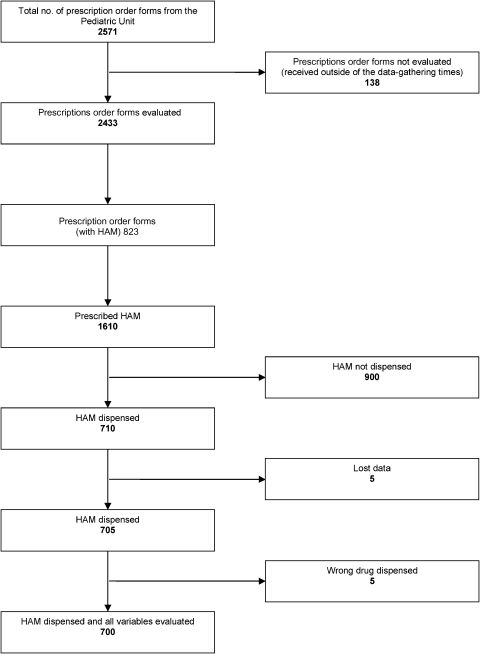
RESULTSDuring the 30-day study period, the dispensing sector received 2,571 copies of prescription order forms for pediatric inpatients. Of these, 2,433 (94.6%) arrived between 7 a.m. and 9 p.m. and thus potentially met our inclusion criteria. Among the 2,433 copies, 823 (33.8%) contained one or more HAMs and were therefore selected for analysis.
Among the 8,875 medications prescribed in the 823 selected prescription order forms, 1,610 (18.1%) were HAMs. Of these 1,610 HAM, 900 (55.9%) were not dispensed, primarily because they were standard inventory medications. The remaining 710 (44.1%) were dispensed.
The 710 dispensed HAMs consisted of 638 (89.9%) medications for individual use and 72 (10.1%) medications from the standard inventory. Five (0.7%) of the 710 prescribed HAMs were dispensed without being evaluated by the researchers and were thus considered to be missing data. Among the remaining 705 HAMs, a drug other than the prescribed medication was dispensed in five instances (0.8%). These instances involved codeine and paracetamol being dispensed instead of codeine, heparin being dispensed instead of enoxaparin, morphine instead of tramadol, and two cases of adrenaline being dispensed instead of noradrenaline. Analysis of the remaining variables related to dispensing errors was therefore possible for 700 HAMs (Figure 1).
There were one or more prescribing errors in 632 (89.6%) of the 705 HAMs that were prescribed and dispensed, totaling 1,632 errors (Table 1). The HAM dose was too high in 33 (4.7%) cases, too low in 10 cases (1.3%), unclear in 319 (45.3%) cases, and absent in 7 (1.0%) cases. The HAM infusion rate was unclear in 110 (20.8% of HAMs administered via infusion) cases and absent in 159 (30.1%) cases. The prescription components that were poorly legible included the name of the HAM (6.7%), its concentration (6.4%), its dose (6.1%), and its infusion rate (5.4%). The lack of clarity in these prescription components created predisposing conditions for potential dispensing errors. Of the 632 HAMs in this study with one or more prescribing errors, the three drugs most frequently associated with prescription errors were injectable midazolam (5 mg/ml) at 22.3% (n = 141), fentanyl (0.05 mg/ml) at 18.4% (n = 116) and morphine (10 mg/ml) at 13.4% (n = 85). The mean number of errors per prescribed HAM was 2.6 (Table 1).
The prevalence of prescribing errors for high-alert medications (HAM) in relation to the prescription components and legibility.
| Error | ||
|---|---|---|
| N | % | |
| Components of prescription order form | 1411 | |
| Name of HAM | 5 | 0.3 |
| Dosage form | 617 | 37.9 |
| Concentration | 38 | 2.3 |
| Administration route | 7 | 0.4 |
| Dose interval | 52 | 3.2 |
| Dose | 369 | 22.6 |
| Infusion rate | 269 | 16.5 |
| Dilution | 5 | 0.3 |
| Denomination of HAM | 49 | 3.0 |
| Legibility | 221 | |
| Poorly legible | 218 | 13.3 |
| Illegible | 3 | 0.2 |
| Total | 1,632 | 100.0 |
There was at least one dispensing error in each HAM dispensed. There were 1,707 dispensing errors in total, of which 768 (45.0%) were content errors, 305 (17.8%) were labeling errors, and 634 (37.2%) were documentation errors (Table 2). Dispensing a HAM without the dosage form being specified on the prescription occurred in 615 (87.9%) of the dispensed HAMs. In addition, 32 (4.6%) of the HAMs were dispensed with a different denomination than that prescribed, without providing information to the staff administering the medication that the drugs were equivalent. All 305 of the HAM-dispensing envelopes had one or more labeling errors.
The prevalence of dispensing error types and subtypes.
| Type of dispensing error | Error | |
|---|---|---|
| N | % | |
| Content | 768 | |
| Dosage form | 615 | 35.9 |
| Concentration | 12 | 0.7 |
| Drug | 5 | 0.4 |
| Dose interval | 21 | 1.2 |
| Quantity (dose) | 77 | 4.5 |
| Dilution/reconstitution | 3 | 0.2 |
| Denomination of HAM | 32 | 1.9 |
| Quality deviation | 3 | 0.2 |
| Labeling | 305 | |
| Labeling issues | 305 | 17.8 |
| Documentation | 634 | |
| Name of person in charge of separation | 634 | 37.2 |
| Total | 1,707 | 100.0 |
Information regarding the name of the person responsible for dispensing the 705 prescribed HAMs was unclear in 263 (37.3%) cases, illegible in 282 (40.0%) cases and absent in 89 (12.6%) cases. The mean number of dispensing errors per HAM was 2.4.
Concomitant prescribing and dispensing errorsAmong the dispensing errors recorded in this study, 723 (42.4%) were concomitant with prescription errors, all of which were content errors (Table 3). There was a statistically significant association (p<0.0001) between these concomitant prescribing and dispensing errors. They were distributed as follows: the dosage form was absent in 613 (84.8%) of the cases; the dose was unclear or absent in 60 (8.3%) of the cases; no dose interval was indicated in 21 (2.9%) of the cases; 15 (2.1%) of the cases involved HAMs being prescribed by their trade or chemical name; the concentration was absent or incorrect in 11 (1.5%) of the cases; the dilution was absent or incorrect in 3 (0.4%) of the cases. Injectable solutions of midazolam (5 mg/ml) and fentanyl (50 mcg/ml) were the HAMs in which concomitant prescribing and dispensing errors occurred most often.
Description and examples of concomitant prescribing and dispensing errors by prescription component.
| Prescription and dispensing components | Error | Examples | |
|---|---|---|---|
| N | % | ||
| Dosage form | 613 | 84.8 | Morphine (10 mg) was prescribed without the dosage form. An injectable solution was dispensed when a tablet was also available at a 10 mg concentration. |
| Concentration | 11 | 1.5 | Fentanyl was prescribed at the incorrect concentration (50 mg/ml). Fentanyl (0.05 mg/ml) was dispensed without asking for concentration confirmation from the prescriber. |
| Dose interval | 21 | 2.9 | Midazolam (5 mg/ml) was prescribed without a definition of the dose interval and dispensed without a confirmation of the dose interval. |
| Dose | 60 | 8.3 | Propranolol (1.6 ml) + distilled water (25 ml) was prescribed; the comma overlapped the line, and the volume was interpreted as 16 ml. Thus, a higher dose was dispensed than that intended by the prescriber. |
| Dilution | 3 | 0.4 | No dilution was prescribed for sodium nitroprussiate, and the drug was dispensed without obtaining confirmation from the prescriber. |
| Denomination of HAM | 15 | 2.1 | Marevan (warfarin) was prescribed but was dispensed as the generic drug with no information about the relation between the trade name and the generic name. This practice is not standard at the institution. |
| Total | 723 | 100 | |
Of the 1,707 dispensing errors that were detected in this study, 723 (42.4%) occurred concomitantly with prescribing errors. This association may be explained by prescription quality being a predisposing factor for dispensing errors.13,19–22
The most frequent prescribing and dispensing error was the lack of a specified dosage form, an error that occurred in 613 (84.8%) of the prescribed HAMs. The second most frequent error was unclear or absent doses, which occurred in 60 (8.3%) of the prescribed HAMs. The third most common error was the lack of a specified dose interval, which occurred in 21 (2.9%) instances and could potentially have resulted in incorrect doses. These results are consistent with other studies of pediatric populations in Brazil and in other countries that have indicated dose-related medication errors as the most frequent errors.6,22–24
These three types of errors represent a major safety concern because patients may receive the wrong doses of these high-alert drugs. Correctly expressing all prescription components is essential for patient safety. Poorly legible prescriptions or omissions of prescription components compromise the efficacy of any medication dispensing system and can result in dispensing errors that lead to inappropriate drug administration.11,13,22,25
An analysis of the HAM nomenclature revealed that the medication trade name was used in 2.1% of cases and that the chemical name was used in 4.8% of cases. A study of prescribing errors in a university hospital in Northeastern Brazil revealed that the medication trade name was used in 30.8% of their cases.26 In contrast, Dean et al.16 conducted a study in England in which the use of a brand name instead of the generic name was not considered a prescription error. The institution in which our study was conducted belongs to the Brazilian public health system, where local regulations mandate the use of generic drug names in prescription order forms.27,28
Among the 11 cases of dispensing errors related to drug concentrations, three involved heparin. In two of these cases, the concentration was absent, and the dispensed concentration was incorrect in the third. In these instances, the person in charge of dispensing dispensed what he or she thought had been prescribed without consulting the prescriber. Rosa et al.13 mention heparin as one of the HAMs associated with a high incidence of prescription errors. Two versions of heparin are available at our study institution; one consists of (5,000 units/ml, in 5 ml vials) and the other has four times this concentration (20,000 units/ml in 0.25 ml vials). Thus, dispensing and administration errors involving this HAMs may cause serious harm.
Respiratory depression, apnea, respiratory arrest, and/or cardiac arrest may occur if inappropriate doses of certain HAMs, such as midazolam, morphine, and fentanyl, are used. It is important to note that these drugs represent some of the HAMs in our study with the highest error prevalences.29,30
We identified situations in which morphine (10 mg) was prescribed without the dosage form being specified, situations in which an injectable solution was dispensed when a tablet was also available at a 10 mg concentration and an example of fentanyl being prescribed at the wrong concentration (50 mg/ml). We also observed an instance of fentanyl (0.05 mg/ml) being dispensed without asking for confirmation from the prescriber.
Involving pharmacists as reviewers of prescription order forms prior to dispensing any medication is an important intervention that promotes detecting and preventing medication errors. Support for the efficacy of this approach has been confirmed in the current literature through reports indicating that this process can help intercept potential problems or errors, thereby preventing harm to patients.30–31
Pediatric patients may also benefit from electronic prescriptions. Several studies have shown that the use of such prescriptions significantly reduces medication errors; the improved organization, clinical support, and legibility of electronic prescriptions are the main reasons.32–33 However, recent studies have also suggested that electronic prescriptions, although they can increase process safety, have their own potential for generating errors as a result of technological and/or organizational factors. Thus, after implementing an electronic system, hospitals should attempt to identify the critical error points and create appropriate preventive measures.34
In our study, the prevalence of HAMs with poorly legible names was 6.7%, which was higher than Rosa et al. 3.7% rate reported in a previous study of a trauma hospital.13 It is possible that this discrepancy is attributable to differing specificities of care in pediatric units. Work efficiency may be compromised when a professional has to spend time deciphering what is written in a prescription, a situation that also likely increases the probability of medication errors.35
Considering the significant rates of prescribing errors that have been found in studies published by Rose et al.13, Neri 26 and Ghaleb et al. 6 as well as the dispensing errors identified by Anacleto et al.11, Costa et al.24 and Beso et al.,18 we can conclude that reducing medication errors should be a priority in implementing a program for the safe use of medication in hospitals.
Health professionals are primarily responsible for preventing medication errors and for promoting safe drug-use practices. However, the pharmaceutical industry, the hospital equipment industry, and the medical device industry can also play a significant role in preventing these types of errors. It is important to highlight that ensuring safe drug-use practices is the responsibility of all participants involved in implementing drug safety.1,3
Recommended activities for increasing the safety of pediatric patients and the efficiency of medication management have been described in the literature. These recommendations include implementing activities to foster good prescription practices, including implementing an electronic prescription system with proper clinical support, having pharmacists review prescription order forms before dispensing medications, and using certain HAMs as triggers for improving the safety in the drug-utilization system.
There are some limitations to this study. For example, the 30-day data collection period may not have been representative of the rest of the year due to the potential confounding effects of seasonal changes in prescribing practices and/or alterations in staff experience. In addition, the data were gathered only between 7 a.m. and 9 p.m.; thus, the 138 (5.4%) prescription order forms that arrived after 9 p.m. were not evaluated. Among these 138 prescriptions, 37 were for HAMs, of which 14 were dispensed. Thus, the sample was largely representative of the studied organization despite not being collected over the entire 24 hours of each day.
The classification of prescribing and dispensing errors that was applied here was adapted to the work processes and standards of the studied institution. Because of the variability in medication use processes that exists between institutions, the generalizability of our results is somewhat compromised. In addition, researcher interference may occur when evaluating prescription legibility, which is a subjective variable.
CONCLUSIONThe concomitant prescribing and dispensing errors found in this study require additional research to explore the relationship between these errors and the causes of these findings.
The dispensing errors encountered in this study underline failures in the dispensing system, which was unable to intercept a significant number of prescribing errors, thereby generating opportunities for errors in drug administration.
The system for prescribing and dispensing drugs in the investigated hospital should be improved by incorporating the best safety practices for preventing medication errors. High-alert medications may be used as triggers for improving the safety of drug-utilization system.
No potential conflict of interest was reported.