The novel coronavirus disease (COVID-19) showed increased morbidity and mortality rates and worse prognosis in individuals with underlying chronic diseases, especially cardiovascular disease and its risk factors, such as hypertension, diabetes, and obesity. There is also evidence of possible links among COVID-19, myocardial infarction, and stroke. Emerging evidence suggests a pro-inflammatory milieu and hypercoagulable state in patients with this infection. Despite anticoagulation, a large proportion of patients requiring intensive care may develop life-threatening thrombotic complications. Indeed, the levels of some markers of hemostatic activation, such as D-dimer, are commonly elevated in COVID-19, indicating potential risk of deep vein thrombosis and pulmonary thromboembolism. In this review, we critically examine and discuss aspects of hypercoagulability and inflammation in COVID-19 and the possible benefits of statins in this scenario, with emphasis on their underlying molecular mechanisms. Moreover, we present recommendations on the use of antiviral drugs in combination with statins.
Coronaviruses have caused two epidemics in the past: severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) (1,2). However, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), designated as coronavirus disease (COVID-19), has since caused an ongoing pandemic that significantly threatens global health (3). Patients infected by SARS-CoV-2 commonly have a series of comorbidities and are susceptible to a wide range of complications (1); among these, coagulation abnormalities and thromboembolic events have been frequently reported, especially in the most critically ill patients (2). In line with this, patients with acute respiratory distress syndrome (ARDS) secondary to COVID-19 are more likely to develop thrombotic complications compared to patients with ARDS of other etiologies (3). These remarkably high prevalence and incidence rates of thrombotic complications have also raised questions about an underlying hypercoagulable state in these individuals. Indeed, increased levels of D-dimer and some pro-inflammatory cytokines are frequently observed in patients with COVID-19 and have shown prognostic significance (4,5). Emerging evidence has also led to the hypothesis that these patients are at higher risk of developing disseminated intravascular coagulation (DIC) (6).
In this review, we will present and discuss clinical and laboratory findings relevant to coagulation abnormalities in patients with COVID-19, incidence of thromboembolic events, and considerations of possible interventions for the management of these complications.
COVID-19 and HypercoagulabilityThe pathogenesis of hypercoagulability in COVID-19 may be related to complement-mediated endothelial injury (7) and increased levels of circulating prothrombotic factors (8). Laboratory parameters from a small cohort of Italian patients with COVID-19 admitted to the intensive care unit (ICU) were consistent with hypercoagulability in association with a severe inflammatory state (9). In another 150 patients referred to ICUs for ARDS, 64 clinically relevant thrombotic events were diagnosed, mainly pulmonary embolism (16.7%) (3). In these patients, von Willebrand factor (vWF) activity and vWF antigen and FVIII levels were increased. More than 95% of patients had elevated D-dimer and fibrinogen levels, and 87.7% tested positive for lupus anticoagulant, a prothrombotic antibody. A reduction in platelet count has also been reported as a marker of worse prognosis, with preliminary studies showing that patients with infection usually develop thrombocytopenia (>36%) (10). In fact, a meta-analysis identified significantly lower platelet counts in patients with severe disease (weighted mean difference, -31 × 103/µL; 95% confidence interval [CI], -35 to -29 ×103/µL), and thrombocytopenia was associated with fivefold odds of having severe disease (odds ratio [OR], 5.1; 95% CI, 1.81 to 14.6) (11). Thrombocytopenia and elevated D-dimer levels can be explained by excessive activation of the coagulation cascade and platelets (12). D-dimer, specifically, is a product of fibrin degradation, and its elevation has been associated with a higher mortality rate, highlighting the important role played by hypercoagulability in the pathophysiology of COVID-19 (13,14). Similarly, D-dimers may stimulate the release of proinflammatory cytokines, such as interleukin 6 (IL-6) (15). These cytokines act in systemic inflammation (16), and there is evidence that elevated interleukin levels can play a role in activating coagulation and are associated with venous thromboembolism (VTE) (17). In a retrospective study, severe cases (n=11) presented higher levels of D-dimer, ferritin, and inflammatory markers, such as IL-6 and tumor necrosis factor alpha (TNF-α), than moderate cases (n=10) (18). Wang et al. (19) analyzed laboratory values of 65 patients with COVID-19 and observed that D-dimer and ferritin levels were higher in those with severe (n=20) and extremely severe (n=15) disease. In a study by Zhou et al. (20), D-dimer levels >1 μg/mL at admission were associated with an increased risk of death (OR, 18.42; 95% CI, 2.64 to 128.55). Moreover, 50% of those who died had coagulopathy compared with 7% of survivors. D-dimer levels >1000 µg/L were associated with a fatal outcome. Likewise, in another study, patients requiring ICU treatment had median D-dimer levels significantly higher than those who did not require ICU admission - 0.5 mg/L (interquartile range [IQR], 0.3-0.8) and 2.4 mg/L (IQR, 0.6-14.4), respectively (21). These findings indicate that D-dimer levels can serve as a prognostic tool for risk stratification in COVID-19.
Due to the aforementioned coagulation abnormalities, many patients with severe COVID-19 may develop life-threatening DIC (6,22). However, the DIC observed in this viral infection has distinct characteristics from that observed in sepsis. Although prolonged prothrombin time, thrombocytopenia, and increased D-dimer level are suggestive of DIC, thrombocytopenia in sepsis is usually more profound; D-dimer levels in patients with COVID-19 do not reach such high levels. Moreover, according to the International Society on Thrombosis and Haemostasis score, most patients with COVID-19 would not be classified as having DIC (4).
Some risk factors and markers of thrombosis and hemostasis in patients with COVID-19 in different studies (10,18–21,23–35) are summarized in Table 1.
Risk factors and characteristics of coagulation and hemostasis markers in patients with COVID-19.
| Studies | N | HTN/CVD (%) | D-dimer (mg/L) (IQR) | Prothrombin time (s) (IQR) | Platelet count (×103/μL) (IQR) |
|---|---|---|---|---|---|
| Chen et al. (18) | 21 | Severe: 36.4/NAModerate: 10/NA | Severe: 2.6 (0.6 to 18.7)Moderate: 0.3 (0.3 to 0.4) | Severe: 14.3 (13.6 to 14.6)Moderate: 13.4 (12.8 to 13.7) | Severe: 157 (134 to 184.5)Moderate: 175.6 (148.3 to 194) |
| Fraissé et al. (24) | 92 | TEV: 62/3No TEV: 65/14 | With TEV: 4.4 (1.8 to 2)Without TEV: 2.2 (1.2 to 5.9) | NA | NA |
| Guan et al. (10) | 1.099 | Severe: 23.7/5.8Nonsevere: 13.4/1.8 | NA | NA | Severe: 137.5 (99 to 179)ϕNonsevere: 172 (139 to 212)ϕ |
| Guo et al. (25) | 187 | Normal TnT: 20.7/3Elevated TnT: 63.5/48.3 | Normal TnT: 0.29 (0.17 to 0.6)Elevated TnT: 3.85 (0.51 to 25.28) | Normal TnT: 12.4 (12 to 13)Elevated TnT: 13.3 (12.2 to 15.3) | NA |
| Han et al. (26) | 94 | NA | Severe: 19.1Nonsevere: 2.1 | Severe: 12.7Nonsevere: 12.2 | NA |
| Huang et al. (21) | 41 | ICU: 15/23Non-ICU: 14/11 | ICU: 2.4 (0.6 to 14.4)Non-ICU: 0.5 (0.3 to 0.8) | ICU: 12.2 (11.2 to 13.4)Non-ICU: 10.7 (9.8 to 12.1) | ICU: 196.0 (165 to 263)Non-ICU: 149.0 (131 to 263) |
| Khamis et al. (27) | 63 | ICU: 29/8.3Non-ICU: 33/5.1 | ICU: 2.3 (0.7 to 4.3)Non-ICU: 0.4 (0.2 to 0.7) | NA | ICU: 253 (213 to 322)Non-ICU: 215 (169 to 264) |
| Liu et al. (28) | 78 | Progression: 9/NAImprovement/stabilization: 18.2/NA | Progression: 0.56 (0.21 to 6.84)*Improvement/stabilization: 0.39 (0.2 to 1.07)* | NA | Progression: 143.9 (64.81†)Improvement/stabilization: 173.2 (55.37†) |
| Pan et al. (29) | 124 | Discharge: 42.9/17.1Nonsurvivor: 52.8/14.6 | Discharge: 1.12 (0.43 to 5.42)Nonsurvivor: 3.97 (0.78 to 8) | NA | Discharge: 197 (143 to 234)Nonsurvivor: 182 (151 to 229) |
| Ren et al. (30) | 48 | All: 39.6/22.9 | All: 3.48 (0.83 to 9.23) | NA | NA |
| Suleyman et al. (31) | 355 | ICU: 78.7/35Non-ICU: 68.7/24.8 | ICU: 1.43 (0.81 to 3.22)Non-ICU: 0.95 (0.57 to 1.57) | NA | NA |
| Tang et al. (32) | 183 | NA | Nonsurvivor: 2.1 (0.8 to 5.3)Survivor: 0.6 (0.4 to 1.3) | Nonsurvivor: 15.5 (14.4 to 16.3)Survivor: 13.6 (13 to 14) | NA |
| Wang et al. (19) | 65 | NA | Mild: 1.6 (3†)Severe: 4.7 (7.4†)Extremely severe: 6.9 (8.4†) | NA | NA |
| Wang et al. (23) | 138 | ICU: 58.3/25.0No ICU: 21.6/10.8 | ICU: 414 (191 to 1324)Non-ICU: 166 (101 to 285) | ICU: 13.2 (12.3 to 14.5)Non-ICU: 12.9 (12.3 to 13.4) | ICU: 142 (119 to 202)Non-ICU: 165 (125 to 188) |
| Wu et al. (33) | 201 | Nonsurvivor: 36.4/9.1Survivor: 17.5/2.5 | Nonsurvivor: 3.95 (1.15 to 10.96)Survivor: 0.49 (0.31 to 1.18) | Nonsurvivor: 11.6 (11.1 to 12.45)Survivor: 11.75 (10.95 to 12.45) | Nonsurvivor: 162 (110.5 to 231)Survivor: 204 (137.25 to 262.75) |
| Yang et al. (34) | 52 | Nonsurvivor: NA/9Survivor: NA/10 | NA | Nonsurvivor: 12.9 (2.9)Survivor: 10.9 (2.7) | Nonsurvivor: 191 (74†)Survivor: 164 (63†) |
| Zhang et al. (35) | 143 | DVT: 42.2/13.6Non-DVT: 36.4/10.4 | DVT: 6.6 (2.5 to 8)Non-DVT: 0.9 (0.4 to 3.5) | DVT: 14.2 (13.3 to 15.4)Non-DVT: 12.9 (12.3 to 14) | DVT: 185.5 (140.3 to 289)Non-DVT: 229 (170.5 to 285.8) |
| Zhou et al. (20) | 191 | Nonsurvivor: 48/24Survivor: 23/1 | Nonsurvivor: 5.2 (1.5 to 21.1)Survivor: 0.6 (0.3 to 1.0) | Nonsurvivor: 12.1 (11.2 to 13.7)Survivor: 11.4 (10.4 to 12.6) | Nonsurvivor: 0.6 (0.5 to 0.8)Survivor: 1.1 (0.8 to 1.5) |
HTN, hypertension; CVD, cardiovascular disease; DVT, deep vein thrombosis; TEV, thrombotic events; TnT, troponin T; ICU, intensive care unit; IQR, interquartile range; NA, not available. †Standard deviation. ϕMedian. *ng/mL.
Besides coagulation abnormalities, severe COVID-19 infection is associated with inflammatory responses, leading to acute lung injury and hypoxemic respiratory failure. This interaction between coagulation and inflammation has been termed thromboinflammation (36). Evidence suggests that patients with ARDS secondary to COVID-19 exhibit extensive systemic inflammation, presenting with an exacerbated release of pro-inflammatory cytokines and chemokines (10).
Moreover, SARS-CoV-2 infection seems to increase C-reactive protein (CRP) levels. A meta-analysis of 38 studies enrolling >3,000 patients found elevated CRP levels in 74% of patients (37). In addition, a multivariate logistic analysis indicated that high CRP levels might be an important risk factor for progression of infection (OR, 10.5; 95% CI, 1.2-34.7) (37). A study conducted in China on 27 patients with COVID-19 showed that CRP levels positively correlated with the diameter of the lung lesion and might reflect the severity of the disease (38). Similar results were observed by Tan et al. (39), who found that CRP levels predicted a more severe form of this disease. Finally, Ruan et al. (40) observed that median CRP levels in COVID-19 survivors were approximately 40 mg/L compared to 125 mg/L in nonsurvivors, suggesting a strong correlation with disease severity and prognosis.
Thrombotic Complications in Patients with COVID-19COVID-19 and Venous ThromboembolismAbnormal coagulopathy tends to occur in most nonsurvivors of COVID-19 (31). It is known that the risk of VTE is increased in critically ill patients requiring transfer to the ICU (41). Thus, it is postulated that this risk would also be high in patients with the most severe forms of COVID-19. Complete autopsy of 12 consecutive SARS-CoV-2-positive individuals showed a high incidence of thromboembolic events (42). Specifically, postmortem examination revealed deep venous thrombosis in 7 of 12 patients (58%), while pulmonary embolism was the direct cause of death in 4 patients. A series of 107 consecutive patients with confirmed COVID-19 who were admitted for pneumonia reported a high incidence of pulmonary embolism during their ICU stay (20.6%), twice as high as the frequency found in the previous year (43). A Chinese study reported that 40% of hospitalized patients with COVID-19 had a high risk of VTE (Padua prediction score ≥4) (44). It is recommended that, in the absence of contraindications, all critically ill patients with COVID-19 receive preventive strategies for VTE (45). Despite this, in a study by Tang et al. (46), of approximately 450 patients with severe COVID-19, only slightly more than 20% received anticoagulant therapy (heparin). As some of these patients developed pulmonary embolism, this may have contributed to a higher mortality rate. In contrast, a French study examined 150 patients with COVID-19 in need of intensive treatment and compared them to patients with non-COVID ARDS. The authors observed that patients with COVID-19 developed significantly more thrombotic complications despite prophylactic or therapeutic anticoagulation (3).
A study on 143 patients hospitalized with COVID-19 showed that 66 (46%) developed deep vein thrombosis (DVT) in the lower limbs. These individuals were older and had lower oxygenation index and higher cardiac injury index. Moreover, those who developed DVT had a higher mortality rate (34.8% versus 11.7%, p=0.001). This study showed that DVT is highly prevalent and associated with adverse outcomes in hospitalized patients with COVID-19 (34).
Because of the importance of thrombotic events in patients with COVID-19, the risk of thrombosis must be carefully assessed. Monitoring platelet count, prothrombin time, activated partial thromboplastin time, and fibrinogen and D-dimer levels every 48h can be an important preventive measure. Age >70 years, prolonged bed rest, postpartum status, use of combined oral contraceptives, and obesity are factors that can contribute to increased thrombotic risk (47).
The prevalence of thromboembolic complications (e.g., DVT, VTE, and pulmonary embolism) in patients with COVID-19 across different studies (29,34,42,43,48–52) is shown in Table 2.
Prevalence of thromboembolic complications in patients with COVID-19.
| Studies | N | Deep Vein Thrombosis - N (%) | Venous Thromboembolism - N (%) | Pulmonary Embolism - N (%) |
|---|---|---|---|---|
| Cui et al. (48) | 81 | NA | Critically ill: 20 (16%) | NA |
| Menter et al. (49) | 21 | NA | NA | Died: 4 (19%) |
| Middeldorp et al. (50) | 198 | NA | All: 19 (20%)Critically ill: 35 (47%)Non-critically ill: 4 (3%) | All: 13 (7%)Critically ill: 11 (15%)Non-critically ill: 4 (2%) |
| Nahum et al. (51) | 34 | Critically ill at admission: 22 (65%)Critically ill 48h after admission: 5 (15%) | NA | NA |
| Poissy et al. (43) | 107 | NA | NA | Critically ill: 22 (21%) |
| Ren et al. (30) | 48 | Critically ill: 41 (85%) | NA | NA |
| Voicu et al. (52) | 56 | Invasive mechanical ventilation: 20 (36%) | NA | NA |
| Wichmann et al. (42) | 12 | Died: 7 (58%) | NA | NA |
| Zhang et al. (35) | 143 | All: 66 (46%) | NA | NA |
NA: Not available.
Patients with preexisting cardiovascular disease have a worse prognosis if infected with SARS-CoV-2. A much higher mortality rate in patients with underlying cardiovascular disease than in the general population was reported (1), reaching almost 20% in hospitalized patients with COVID-19 (53). These patients have a higher risk of developing cardiac injury (24), being critically ill (9), and requiring intensive care (53). A retrospective series of 187 COVID-19 cases reported a 7.6% mortality rate for patients without underlying cardiovascular disease and normal troponin levels compared to almost 70% for those with cardiovascular disease and high troponin levels (24). However, it is important to note that, even with normal troponin levels, the presence of underlying cardiovascular disease is associated with a non-negligible increase in the risk of death. As an example, the study by Guo et al. (25) involving 187 patients reported a mortality rate of 13%.
Postmortem studies in Switzerland found a high prevalence of pulmonary embolism with microthrombi in the alveolar capillaries (45% of those with available histology). More than 70% of these patients had underlying chronic diseases, such as cardiovascular disease (48). Similar data were reported by Wichmann et al. (42) in autopsies of patients with COVID-19, where deep vein thrombosis and preexisting chronic diseases were both highly prevalent (58% and 50%, respectively). Although these autopsy findings should be interpreted with caution, due to the small sample size, the high incidence of thromboembolic events in individuals with preexisting cardiovascular diseases suggests a possible relationship between COVID-19-induced coagulopathy and thrombosis with these diseases.
COVID-19 and Myocardial Infarction (MI)The COVID-19 pandemic has caused a significant reduction in the number of cardiac interventional procedures. In Spain, for example, cardiac catheterizations fell by almost 50% (54); similar data were reported in the United States (55). The association between acute infections and increased risk of type 2 MI suggests a causal relationship. The risk of MI associated with pneumonia seems to peak at the onset of this infection and is proportional to disease severity (56). Interestingly, Kwong et al. (57) studied patients infected with influenza virus and its association with hospitalizations for MI that occurred within 1 year before or after its diagnosis. Within 7 days after detection of influenza B, influenza A, respiratory syncytial virus, and other viruses, the incidence rate of MI was >10 times (95% CI, 4.37-23.38), 5 times (95% CI, 3.02-8.84), 3.5 times (95% CI, 1.11-11.12), and 2.77 times (95% CI, 1.23-6.24) higher, respectively, than that at baseline, suggesting an association between respiratory infections and MI. A US case series has shown that the risk of MI increases exponentially during the first 15 days after hospitalization for acute bacterial pneumonia, with risk being almost 50-fold greater than in any 15-day period during the year before or after the onset of infection (58).
ST segment elevation on electrocardiogram has been reported in a series of patients with COVID-19 from New York. Importantly, although 18 patients presented with high D-dimer levels, 8 patients with clinical diagnosis of MI presented with median levels higher than those of 10 patients with non-coronary myocardial injury - 1,909 ng/mL (IQR, 682-19,653) versus 858 ng/mL (IQR, 541-3,580), respectively (59). In a study of patients undergoing primary percutaneous coronary intervention for ST-Elevation Myocardial Infarction (STEMI), high D-dimer levels at admission were associated with larger MI size, greater extent of area at risk, and lower myocardial salvage index, suggesting that D-dimer levels may be a marker of advanced myocardial injury (60).
What are the possible hypotheses that may explain an increased risk of MI in patients with COVID-19? With the release of pro-inflammatory cytokines and catecholamines, compounded by increases in both oxygen demand and heart rate caused by the infection, a marked reduction in coronary perfusion is observed (56). These and other factors may contribute to a mismatch between oxygen demand and oxygen supply, resulting in ischemia, injury, and fibrosis.
COVID-19 and ObesityPatients with COVID-19 who are overweight or obese may also be more susceptible to complications. A study enrolling 775 infected individuals showed that 72% were overweight or obese. Among those with a body mass index (BMI) >30 kg/m2 who were admitted to the ICU, 61% died (61). In a French retrospective study (n=124), 48% of patients were obese, and 28% were severely obese (BMI >35 kg/m2). As the BMI category increased, patients required more invasive mechanical ventilation (62). Similar data were observed in a study by Hu et al. (63) in China (n=58). The proportion of prolonged hospital stay was significantly higher in overweight or obese patients than in those with normal weight (62.1% versus 26.1%, p=0.01). In addition, discharge from the hospital was inversely and independently associated with BMI (hazard ratio [HR], 0.75; 95% CI, 0.63-0.90).
Some factors have been speculated to increase morbidity in obese patients with COVID-19. Increased levels of pro-inflammatory cytokines, such as TNF-α and IL-6, may be more common in overweight and obese individuals (64). This pro-inflammatory environment, in association with compromised fibrinolysis, may contribute to increased thrombotic risk, which may be associated with worsening lung damage (65). Moreover, as obese patients commonly have other comorbidities at baseline, these may represent an additional risk factor for COVID-19 complications. Finally, adipose tissue itself has been demonstrated to serve as a reservoir for several types of viruses, such as human adenovirus Ad-36, influenza A virus, and cytomegalovirus (66). By analogy, COVID-19 may also affect the adipose tissue and subsequently compromise other organs.
Prevention of Venous Thromboembolism in Patients with COVID-19It is known that patients with COVID-19 have an increased risk of thrombotic events (42-44). These individuals may also have increased presence of extramedullary megakaryocytes in organs such as the lungs and heart. This is a phenomenon that seems to play an important role in increasing thrombosis in these patients (67).
Klok et al. (68) studied 184 patients with COVID-19 who required ICU admission and found a 31% incidence of thrombotic complications. In turn, Tang et al. found lower 28-day mortality in heparin users - unfractionated heparin or low molecular weight heparin (LMWH) - compared to that in non-users in the subgroup with sepsis-induced coagulopathy (SIC) score ≥4 (40.0% versus 64.2%; p=0.029) or D-dimer level of more than sixfold of the upper limit of normal (32.8% versus 52.4%; p=0.017), suggesting a better prognosis in patients who met the SIC criteria or with markedly high D-dimer levels (46).
Given the clinical importance associated with a high risk of VTE in patients with severe COVID-19, antithrombotic preventive therapy is strongly recommended in the absence of contraindications (69-73). Patients with contraindications to anticoagulants should be treated with limb compression (69). Several recommendations for prophylaxis for the prevention of VTE in patients with COVID-19 were published (69-73). Many of these patients may have high Padua scores and reduced mortality rates with the use of LMWH or unfractionated heparin (72).
European authors recommend that in patients with COVID-19 and D-dimer levels <0.5 μg/mL, prophylactic-dose anticoagulation is recommended (enoxaparin 40 mg every 24h); if D-dimer level is between 0.5 and 3.0. μg/mL, enoxaparin 40 mg every 12h; if D-dimer level is >3.0 μg/mL, enoxaparin 1 mg/kg every 12h (73). Some authors suggest a more aggressive prophylactic therapy (e.g., enoxaparin 30 mg subcutaneously every 12h for those with body weight ≤120 kg and 40 mg subcutaneously every 12h for patients with body weight >120 kg) (74).
The American College of Chest Physicians recommends that hospitalized patients with COVID-19 receive anticoagulant thromboprophylaxis with LMWH or fondaparinux over unfractionated heparin; direct-acting oral anticoagulants are not indicated. In patients with severe COVID-19, standard-dose thromboprophylaxis is suggested over the intermediate one (LMWH every 12h or increased weight-based dose) (70).
The International Society on Thrombosis and Haemostasis suggests that a half-therapeutic-dose of LMWH (1 mg/kg every 24h) can be considered for high-risk patients with COVID-19 and a 50% higher dose should be considered for obese individuals (75). In addition, also recommends thromboprophylaxis with LMWH or a direct-acting oral anticoagulant (duration of 14 to 30 days) after discharge from those patients considered at high risk (e.g., age >65 years, cancer, previous VTE, elevated D-dimer level (>2 times the upper limit of normal, etc.), and low risk of bleeding (76). In contrast, thromboprophylaxis for patients who do not require hospitalization is not currently recommended (76).
In an editorial published by Lopes and Fanaroff, the authors emphasize the importance of conducting randomized clinical trials in the COVID-19 anticoagulation scenario. They pointed out that the correct dose and best type of anticoagulant for these patients must be better investigated (77).
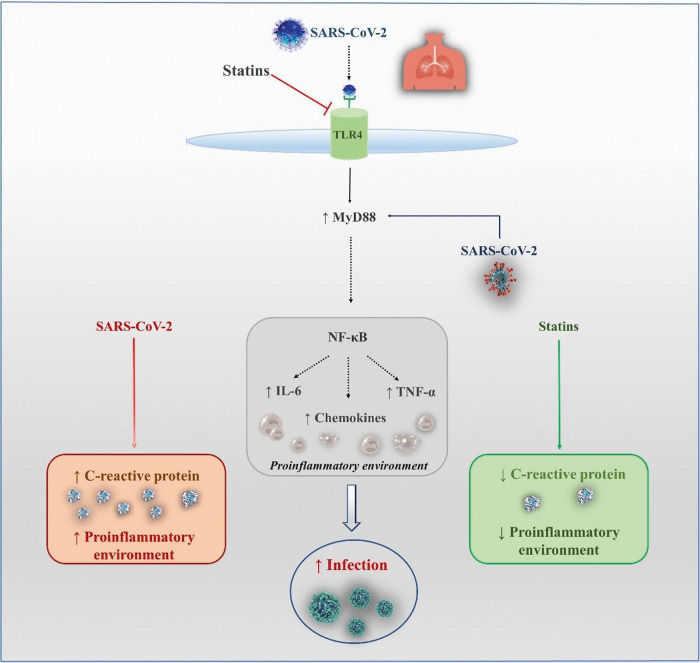
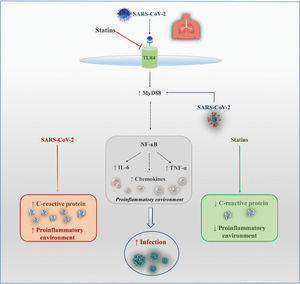
Possible Benefits of Statins in COVID-19Anti-inflammatory and Antithrombotic EffectsSeveral mechanisms have been proposed to support the hypothesis of a protective effect of statins, as observed in studies of patients with influenza or COVID-19 (Figure 1). Reduction in inflammation via inhibition of nuclear factor kappa B (NF-κB) may be one of these components. In an experimental study (78), NF-κB inhibition led to a reduction in inflammation and lung injury and significantly increased survival after SARS-CoV-2 infection. Moreover, Bahrami et al. (79) suggested that statins could also have a positive impact on inflammatory diseases by reducing toll-like receptor 4 (TLR4) expression. Statins can also regulate the TLR4/myeloid differentiation primary response 88 (MyD88)/NF-κB signaling pathway. MyD88 is an essential adaptive molecule for signaling through TLRs, resulting in signal transduction and production of pro-inflammatory cytokines. As it has been observed that SARS-CoV infection can induce expression of the MyD88 gene, the ability of statins to maintain MyD88 at normal levels may be protective for patients with COVID-19. This balance is important because both overexpression of MyD88 and its deficiency led to increased mortality rates after MERS-CoV infection (80). However, a statin-induced increase in IL-18 levels might create an unfavorable environment in SARS-CoV-2 infection and even increase the disease severity (81).
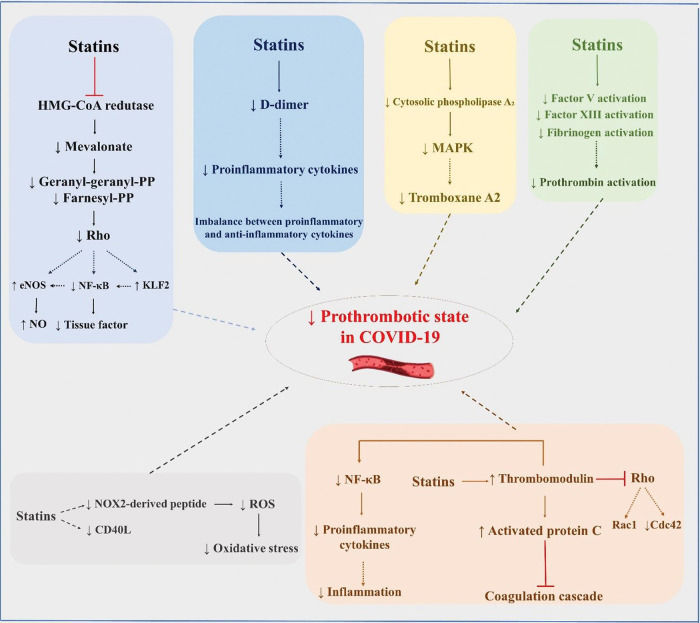
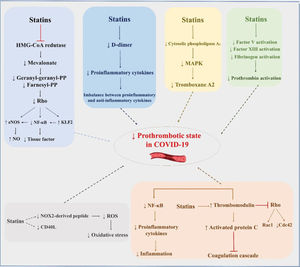
Statins may also exert favorable effects in COVID-19 through their anti-thrombotic effects (Figure 2). More than 20 years ago, Colli et al. (82) demonstrated that statins can have protective effects against thrombotic events. In another study, after vascular injury, early statin therapy was found to act on platelet cytosolic phospholipase A2 activity through its effects on mitogen-activated protein kinases and calcium, reducing thromboxane A2 synthesis by approximately 30% (83). An ex vivo study with 10 patients with hypercholesterolemia showed that, at 2h after administration of 20 mg rosuvastatin, recruitment of platelets, platelet CD40L, and soluble NOX2-derived peptide decreased, while platelet nitric oxide increased. In the group that received only dietary intervention, no change was observed. These findings suggest that rosuvastatin promotes a reduction in oxidative stress and platelet activity (84).
Possible pleiotropic effects of statins to reduce thrombotic complications in patients with COVID-19. eNOS, endothelial nitric oxide synthase; NF-κB, nuclear factor kappa B; KLF2, Krüppel-like factor 2; NO, nitric oxide; MAPK, mitogen-activated protein kinase; ROS, reactive oxygen species.
Bruni et al. (85) demonstrated that the administration of atorvastatin and simvastatin significantly reduced P-selectin, CD36, and LOX-1. Undas et al. (86) reported antithrombotic effects at sites of microvascular injury with a 3-month course of simvastatin in patients with coronary artery disease. Attenuation of the activity of factor V, factor XIII, fibrinogen, and prothrombin is another potential mechanism of antithrombotic action of statins, regardless of cholesterol level reduction. Statins can also increase the expression of thrombomodulin and activated protein C, inhibiting the coagulation cascade (87). Increases in mRNA levels of thrombomodulin inhibit geranylgeranylation of proteins in the Rho subfamily (e.g., Rac1/Cdc42) and, consequently, activation of NF-κB (88), promoting a less inflammatory environment. In addition to the possible pleiotropic anti-inflammatory and immunomodulatory effects of statins, which may be beneficial in COVID-19, Reiner et al. (89) suggested, based on an in silico molecular docking experiment, that statins may inhibit the main SARS-CoV-2 protease. This raises the hypothesis that statins may be directly beneficial against SARS-CoV-2 infection through a novel mechanism.
In 2012, Vandermeer et al. (90) included data from >3,000 patients hospitalized for influenza, one-third of whom received statin therapy. Using a multivariable logistic regression model, the authors found that statin therapy before or during hospitalization was associated with a lower risk of mortality (OR, 0.59; 95% CI, 0.38-0.92). In another study, patients with viral pneumonia (n=539) on continuous use of statins presented lower rates of death and intubation during hospitalization (OR, 0.26; 95% CI, 0.08-0.81). Therefore, patients admitted with viral respiratory diseases, including COVID-19, may benefit from therapy with these drugs. However, these findings should be interpreted with caution due to the limitations inherent to observational studies.
A meta-analysis of nine randomized clinical trials (n=1,165) showed that statins can significantly reduce plasma D-dimer levels (SMD -0.988 µg/mL; 95% CI, -1.590 to -0.385). A subgroup analysis of patients on atorvastatin and simvastatin indicated that this effect was significant only with at least 12 weeks of treatment (91). These findings suggest a potential role of statins in reducing coagulation and preventing VTE.
Zhang et al. (92) suggested a potential reduction in all-cause mortality in patients with COVID-19 who received statin therapy. In this retrospective study performed in China, approximately 14,000 patients were included, of whom 1,219 received statin therapy. Atorvastatin was the most commonly used statin (83%), followed by rosuvastatin (15%). These individuals had lower crude mortality at 28 days compared to those without statin use (incidence rate ratios, 0.78; 95% CI, 0.61-0.996). When adjustments for baseline differences were analyzed, statin therapy was also associated with lower mortality (adjusted HR, 0.63; 95% CI, 0.48-0.84; p=0.001). Another study observed a slower progression to death in ICU patients with COVID-19 using atorvastatin (93). Although Kow and Hasan's meta-analysis has shown a reduction in the risk of fatal or severe disease with the use of statins (HR, 0.70; 95% CI, 0.53-0.94) compared to that with the non-use of statins in patients with COVID-19 (94), another meta-analysis did not observe improvement in disease severity (OR, 1.64; 95% CI, 0.51-5.23; p=0.41), nor in the mortality rate by COVID-19 (OR, 0.78; 95% CI, 0.50-1.21; p=0.26) (95).
Despite indirect evidence pointing to the potential benefit of statins in patients with COVID-19, there is a lack of robust clinical evidence on their role in reducing clinical outcomes.
Prevention of Venous Thromboembolism with StatinsVTE has a significant impact on the patient's health costs, morbidity, and mortality. In the absence of adequate medical treatment, VTE can reduce blood flow and oxygen demand, damaging organs and tissues (96).
In a prespecified analysis of the JUPITER trial, the authors evaluated the impact of rosuvastatin on the first occurrence of VTE. During a median follow-up of 1.9 years (maximum 5.0), there were 34 occurrences of symptomatic VTE in the rosuvastatin group and 60 in the placebo group, corresponding to a 43% reduction in the VTE rates in favor of the statin group (HR, 0.57; 95% CI, 0.37-0.86; p=0.007) (97). Stewart et al. (98) evaluated the impact of statin treatment on VTE recurrence in a cohort of 192,908 patients (mean age, 67 years) recruited between 2004 and 2017. After propensity matching, the group of patients who did not use statins presented higher rates of VTE recurrence compared to statin users (20% versus 16%; p<0.0001). After adjustments for a series of variables, a 25% reduction in VTE recurrence was observed in patients receiving statin (OR, 0.75; 95% CI, 0.72-0.79).
These data were analyzed by meta-analyses. In one of them, in the risk analysis of VTE development (one randomized clinical trial and eight observational studies; n=845.445), it was reported that the use of statins reduced the odds of developing VTE by 32% (adjusted OR, 0.68; 95% CI, 0.54-0.86) (99). In a subsequent meta-analysis, Kunutsor et al. (100) compiled 13 cohort studies (n=3,148,259) and 23 randomized clinical trials (n=118,464) and analyzed them separately. The authors found a reduction in the risk of VTE when the statin group was compared to the control group (relative risk [RR], 0.75; 95% CI, 0.65-0.87; p<0.0001; 0.85; 95% CI, 0.73-0.99; p=0.038, respectively). In addition, rosuvastatin showed greater benefit over the risk of VTE compared to other statins (RR, 0.57; 95% CI, 0.22-0.75; p=0.015).
These data should be viewed with caution given their potential biases (101), and randomized clinical trials are needed to assess the real effectiveness of statins in VTE prevention.
Drug-Drug Interactions with Antiviral TherapyIt is important to mention that caution should be taken when administering statins (102) concomitantly with antiviral therapy in patients with COVID-19 (Table 3).
Concomitant use of antivirals and statins in patients with COVID-19.
| Drug | Use with lopinavir/ritonavir | Rationale |
|---|---|---|
| Atorvastatin | Can be administered | Prefer the lowest possible dose |
| Lovastatin | Contraindicated | Increased risk of rhabdomyolysis |
| Rosuvastatin | Can be administered | Prefer the lowest possible dose |
| Simvastatin | Contraindicated | Increased risk of rhabdomyolysis |
CYP450, cytochrome P450; BID, twice daily.
COVID-19 is associated with a hyperinflammatory and hypercoagulable state, especially in patients with severe disease, which results in an increased risk of thrombotic complications. Patients with preexisting cardiovascular disease and those with cardiovascular risk factors seem to be at high risk for severe complications of SARS-CoV-2 infection. Although statins have established anti-inflammatory and antithrombotic effects, there is no clear evidence that these drugs reduce morbidity and mortality in patients with COVID-19. Therefore, their use needs to be further explored through the conduction of high-quality randomized clinical trials.
Conflicts of Interest. The author RDS has received honoraria for consulting, research, and/or speaker activities from Aché, Amgen, AstraZeneca, Esperion, Kowa, Novo Nordisk, Merck, MSD, Pfizer, PTC, and Sanofi/Regeneron.
AUTHOR CONTRIBUTIONSFerrari F, Martins VM, Santos RD and Stein R were responsible for the research design and conception. Ferrari F, Martins VM, Teixeira M, Santos RD and Stein R were responsible for the data acquisition, analysis and interpretation, and manuscript writing. Ferrari F, Santos RD and Stein R were responsible for the critical revision of the manuscript on important intellectual content.
RDS is the recipient of a Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq) research grant (#303734/2018-3). This study was partially supported by the Hospital de Clínicas de Porto Alegre Research Incentive Fund (FIPE-HCPA), Porto Alegre, Brazil. FF and MT receive financial support from the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES).