Cancer is the third most frequent cause of death in children in Brazil. Early diagnosis and medical advances have significantly improved treatment outcomes, which has resulted in higher survival rates and the management of late side effects has become increasingly important in caring for these patients. Dental abnormalities are commonly observed as late effects of antineoplastic therapy in the oral cavity. The incidence and severity of the dental abnormalities depend on the child's age at diagnosis and the type of chemotherapeutic agent used, as well as the irradiation dose and area. The treatment duration and aggressivity should also be considered. Disturbances in dental development are characterized by changes in shape, number and root development. Enamel anomalies, such as discoloration, opacities and hypoplasia are also observed in these patients. When severe, these abnormalities can cause functional and esthetic sequelae that have an impact on the children's and adolescents' quality of life. General dentists and pediatric dentists should understand these dental abnormalities and how to identify them aiming for early diagnosis and appropriate treatment.
Cancer is the third most frequent cause of death in children in Brazil, after accidents and violence (1). The survival rates of childhood cancer have significantly increased due to early diagnosis and advances in medicine, so attention has become focused on the late effects of antineoplastic therapy.
Dental anomalies are among the most common long-term side effects of childhood cancer therapy in the oral cavity. They may lead to anatomic, functional and aesthetic sequelae, and severe abnormalities can cause malocclusion, affect facial development and impact the quality of life (2).
The incidence and severity of dental abnormalities depend on the age at the diagnosis and the type of chemotherapeutic agent used, as well as the irradiation dose and area (3,4). The duration and severity of antineoplastic treatment should also be considered. Dental development disturbances are characterized by changes in shape, size, number and root development.
General dentists and pediatric dentists provide oral care to childhood cancer survivors. Therefore, it is imperative that they familiarize themselves with the adverse effects of cancer therapy.
Epidemiology of childhood cancerMore than 9,000 new childhood cancers are diagnosed annually in Brazil. Cancer is the third leading cause of death in the 1- to 19-year-old age group, behind accidents and violence.
Childhood cancer requires special care for not only the psychological and social effects but also because of the high costs involved in diagnosis, treatment and long-term follow-up (5). The types of cancers that affect children younger than 15 years old are distinct from those that affect adults. The most prevalent childhood cancers are leukemias, lymphomas, central nervous system tumors, rhabdomyosarcoma, Wilms' tumor, retinoblastoma and bone tumors; while in adults, lung, stomach, intestine, prostate and breast cancer predominate (6). The literature reports that during childhood, acute lymphocytic leukemia (ALL) is the most common malignancy, representing 24% of all childhood malignant neoplasias (7-9) and 75% of all childhood leukemias (10). The 0- to 4-year-old age group is cited as the age group most frequently affected by cancers; however, lymphomas, carcinomas and bone tumors are the most prevalent cancers in the 10-14-year-old age group (11,12). In Brazil, leukemia predominates in the 1- to 4-year-old age group (31.6%); lymphoma is dominant in the 15- to 18-year-old age group (35.6%), and central nervous system tumors have a similar prevalence (26%) in all patients younger than 14 years old (1 to 4, 5 to 9, and 10 to 14 years old)(1). With regard to gender, related studies agree that general tumors, leukemias, lymphomas and central nervous system tumors are more prevalent in males than in females (5).
Late effects of childhood cancer therapyWith the increasing number of childhood cancer survivors comes a high incidence of adverse effects because of more aggressive treatments protocols. Therefore, increasing focus has been directed toward the late sequelae of treatment modalities.
The combination of surgery, chemotherapy and radiotherapy is extremely effective not only in prolonging life but also in obtaining significant cure rates in many types of childhood cancer (13). In Brazil, the survival rates range from 70 to 80% in some types of childhood cancer, such as ALL (1).According to Dickerman (2007)(14), the number of long-term survivors of childhood cancer continues to increase and approximately 75% will have a chronic health problem resulting from the cancer therapy, whereas 40% will experience a severe, disabling, or life-threatening condition or death caused by a chronic condition.
A variety of late effects of childhood cancer therapy can be noted as malignant late effects (secondary cancer) and nonmalignant late effects, in which many organs can be affected, including the oral cavity (13,14)(Table 1).
Prevalence of dental anomalies in childhood cancer survivors.
| Authors | N | Diagnosis | GenderF M | Mean age | Dental anomalies | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hypoplasia | Microdontia | Hypodontia | Taurodontia | Tapered roots | Blunted roots | |||||
| Rosenberg et al. 1987 | 17 | ALL | _ _ | 7.2 | _ | _ | _ | _ | 1376% | 529% |
| Pajari et al. 1988 | 34 | Various tumors | 19 18 | 5.7 | 3294% | _ | _ | _ | _ | _ |
| Dahllof et al. 1988 | 16 | BMT | 7 9 | 7.1 | 425% | 319% | _ | _ | 1488% | 531% |
| Sonis et al. 1990 | 97 | ALL | 61 36 | _ | 2728% | 2122% | 55% | _ | 8588% | 6062% |
| Nunn et al. 1991 | 52 | ALL+lympho-ma | 30 22 | 6.7 | 1427% | 1427% | 815% | 1427% | 1427% | 1427% |
| Pajari et al. 1995 | 45 | ALL | 25 20 | 5.4 | 4095% | _ | _ | _ | _ | _ |
| Kaste et al. 1995 | 22 | Rhabdomyo-sarcoma | 12 10 | 5.1 | _ | 523% | 1150% | _ | _ | 1359% |
| Kaste et al. 1997 | 426 | ALL | 204 259 | 4.8 | _ | 8019% | 369% | 256% | _ | 10324% |
| Nasmann et al. 1997 | 16 | BMT+TBI | 9 7 | 6.3 | 744% | 1275% | 956% | _ | 1594% | 1169% |
| 52 | BMT no TBI | 23 29 | 5.1 | 713% | 713% | 1121% | _ | 1019% | 48% | |
| Kaste et al. 1998 | 52 | Neuroblastoma | 9 33 | 0.0 | 917% | 2038% | 917% | _ | _ | 917% |
| Aspalan et al. 1999 | 30 | Lymphoma | 7 23 | _ | 1447% | _ | 1550% | _ | 930% | 27% |
| Minicucci et al. 2003 | 76 | ALL | 33 43 | 5.1 | 2539% | 2844% | _ | _ | _ | _ |
| Lopes et al. 2006 | 137 | Various tumors | 79 58 | 5.6 | _ | 97% | 86% | 1914% | 22% | 54% |
Complications resulting from the cancer itself and from its treatment frequently affect the mouth. Chemotherapy and head and neck radiotherapy mainly affect developing tissues, such as teeth and oral soft tissues (15). Generally, many therapeutic modalities are used to treat childhood cancer. Chemotherapy and radiotherapy are often combined, making it difficult to later distinguish which treatment modality causes which effects (16). Oral late effects of cancer therapy are clinically significant because of the sequelae they can cause, which may interfere with the quality of life. The most commonly observed late effects of head and neck radiotherapy therapy include xerostomia, trismus, bone alterations that can cause osteoradionecrosis and craniofacial and dental anomalies (17-19). Dental anomalies are also caused by chemotherapy; the main late effects of this therapy are in the mouth (3,16,20).
Dental anomalies in children submitted to antineoplastic therapyAntineoplastic therapy can cause disturbances in tooth eruption and development. The exact molecular mechanisms of antineoplastic therapy that result in dental anomalies remain unknown (21,22).
The lack of specificity of both chemotherapeutic agents and radiation therapy in terms of differentiating neoplastic cells from metabolically active normal cells might result in dental and facial development abnormalities (3). The severity of these effects on dentofacial structures was found to be related to the stage of tooth histogenesis, age at diagnosis, type of treatment performed and irradiation dose area (3,21). Studies (3,4,16), have shown a direct relationship between the severity and prevalence of dental anomalies and age at diagnosis, which is related to odontogenesis stage. Children treated before 5 years of age had the most severe dental defects, suggesting that immature teeth were at greater risk for developmental disturbances than mature teeth (3). With the increased life expectancy of patients younger than 5 years old, it is important to study late dental effects in children who undergo cancer treatment at such an early age (16). Radiotherapy can cause disturbances in dental development in children; however, the minimal radiation dose necessary to cause changes in dental development is unknown. According to Carpenter (27) and Dury (28), 1,000 cGy is sufficient to cause permanent damage to mature ameloblasts and 3,000 cGy is sufficient to arrest dental development. However, Fromm et al. (29) and Goho (2) identified alterations in dental development after 400 cGy dose.
Many authors (16,23,24,30) have reported a higher prevalence of (and more severe) dental anomalies in children submitted to head and neck radiotherapy and total body irradiation combined with chemotherapy compared to children submitted to only chemotherapy. Raney et al. (31) and Paulino et al. (32) reported that 75-100% of patients submitted to radiotherapy presented light to mild hard and soft tissue damage resulting from irradiation.
The extent and severity of the defects is related to the radiation type and dose, the age of the patient, irradiation area, degree of tissue hypovascularity and hypocellularity, reparative capacity of epithelial cells and associated chemotherapy (31,33-35). Animal studies (36,37) have shown dental development disturbances induced by vincristine, vinblastine, doxorubicin and cyclophosphamide. The extent of dental abnormalities attributed to chemotherapy depends on many factors, such as the type of chemotherapeutic agent used, half-life of the drug and number of cells in susceptible phases of the cell cycle (37). In addition, the combination of several agents in chemotherapy protocols, often combined with radiation therapy, makes it difficult to attribute anomalies in odontogenesis to any single agent or therapy in these cases and to determine how they interfere in cellular physiology (37).
Depending on the odontogenesis stage, certain types of anomalies can be observed (38). Therefore, detailed knowledge of tooth development by dental professionals is fundamental in interpreting the timing and nature of dental developmental anomalies and consequently correlating them to the age at which the event occurred. In patients submitted to antineoplastic therapy, this correlation is easily observed, reinforcing the premise that dental development represents a real biological milestone in health and illness as well as in the relationship between the mouth and many diseases and their manifestations and treatments.
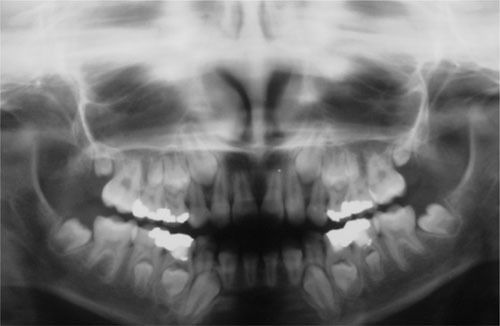
Antineoplastic therapies that affect the cells involved in odontogenesis may cause changes in tooth enamel and root development, premature apex closure, dental development delay or retained teeth (3,16,21,24,26). Dental anomalies of shape (microdontia, macrodontia and taurodontia) and number (hypodontia), enamel defects (discolorations and hypoplasia) and root formation disturbances (blunted root, tapered root and root development delay) have been described in many studies (3,16,24,26,39). Other anomalies, such as supernumerary teeth, have also been detected (24,26). Developmental defects of the enamel organ are well documented in the scientific literature. Enamel hypoplasias and discolorations are the most frequent dental defects from cytotoxic drugs (29,30,37,40). Children treated for ALL seem to be more severely affected and this result may be reflected in the longer duration of therapy, which leads to a greater risk of affecting the developing ameloblasts (24,41,42). Disturbances in dental morphology induced by high-dose chemotherapy and total body irradiation in patients have been shown in many histologic studies. These studies have also shown that radiation therapy induces both quantitative and qualitative changes in enamel and dentin during tooth formation, whereas chemotherapy induces mostly qualitative disturbances (24,42). Microdontia is frequently detected in long-term childhood cancer survivors. Several studies have reported a prevalence of microdontia ranging from 22-78% in this population (3,43,44). Clinically, hypodontia and microdontia have great importance because they can cause spacing and movement of teeth, resulting in poor dental alignment and malocclusion (45) (Figure1).Intensive and repetitive chemotherapy during initial hard tissue formation can cause hypodontia (39,42), which consists of the absence of one or more teeth. It is caused by disturbances in the first periods of the tooth vital cycle because of the failure of the dental lamina and tooth bud development processes or because of impairment of the cellular multiplication that promotes the development of these buds (proliferation stage)(38).
For antineoplastic therapy to result in dental agenesis, it must destroy the cells programmed to form a tooth or to affect the signaling systems between the tissues in a tooth bud and prevent calcification (43). Some studies have reported a prevalence of hypodontia associated with anticancer therapy ranging from 6 to 44% (16,23,25,44). The effects of cancer treatment in the later stages of tooth formation are characterized by disturbed root development (43). The first signs of root development in permanent teeth are observed on panoramic radiographs beginning approximately at age 3 years (central incisors and permanent first molars) to age 7.5 years (permanent second molars) (43) and root formation is completed after the tooth has erupted into the oral cavity (46). Changes in odontoblast activity caused by abnormal secretory functioning of the dentin microtubules and complex changes in inter- and intracellular relationships can produce shortened, tapered and blunted roots (42) (Figure3).
Root development disturbances, such as arrested root development with short V-shaped roots, arrested root development with premature apical closure, blunted roots and anomalies in root number have been reported in patients treated with high-dose chemotherapy and radiotherapy (3). Repeated high-dose chemotherapy can result in root agenesis (42).
Another dental anomaly that is frequently detected in these patients is taurodontia (Figure2), which is characterized by an enlarged pulp chamber, apical displacement of the pulpal floor and no constriction at the level of the cementoenamel junction (47), which is caused by the failure of Hertwig's epithelial sheath diaphragm to invaginate at the proper horizontal level (48). This abnormality usually affects permanent molar teeth, but it can also be observed in premolars and primary molar teeth and can become a challenge when endodontic treatment is needed, particularly because of its anatomy (45). In the dental literature, its prevalence in children treated for childhood cancer varies from 6% to 26% (16,45,46).
Many studies have shown differences in the prevalence of dental anomalies in long-term survivors of childhood cancer, most likely because of the type of tumor studied, the chemotherapy protocol used and the age of the study population, as well as associated body and/or head and neck irradiation and different assessment methodologies.
Unfortunately, few studies in the scientific literature that have described the prevalence of dental anomalies in healthy children in the Brazilian population. These studies are essential as a comparative measure not only for children submitted to antineoplastic therapy but also for children who present other chronic diseases that affect dental development. However, several studies have demonstrated a high prevalence of dental anomalies in children submitted to cancer therapy compared to healthy children (16,23-26,45,49).
Children submitted to antineoplastic treatment present several late effects in many organs and systems, including the oral cavity; these effects are caused by anticancer treatments. Dental abnormalities are the most frequent sequelae of therapy for childhood cancer; these abnormalities include microdontia, hypodontia and enamel and root development disturbances. These abnormalities can cause malocclusion and affect facial development, consequently impacting quality of life. Children who are treated at young ages appear to be more severely affected than children who are treated later; additionally, radiotherapy seems to cause more extensive and severe dental defects compared to chemotherapy. Considering these factors, general and pediatric dentists should become familiarize themselves with these late effects, aiming for early diagnosis, proper dental care and, consequently, improved quality of life for this increasingly large group of children.
AUTHOR CONTRIBUTIONSCarrillo CM was responsible for literature review and manuscript writing. Corrêa FN was responsible for the first manuscript revision. Lopes NN was responsible for the images and manuscript review. Fava M was responsible for literature review and manuscript evaluation. Odone Filho V was responsible for final revision.
No potential conflict of interest was reported.