To analyze angiotomographic parameters of juxtarenal aneurysms to assess the applicability of an endograft model to patients and to create in vitro and in vivo models to assess the new endograft.
METHODS:A total of 49 patients with juxtarenal aneurysms were submitted to angiotomographic evaluation, and parameters such as the aortic diameter, the length of the neck, and the angulations of the celiac trunk, superior mesenteric artery and renal arteries; the distances between them; and anatomic variations were analyzed. Based on these parameters, an endograft model was developed and tested in a newly created in vitro model of juxtarenal aneurysm. An experimental model of juxtarenal aneurysm was then established in six pigs weighing 50–60 kg to assess the new endograft model.
RESULTS:The angiotomographic parameters of juxtarenal aneurysm measured in this study were similar to those reported in the literature and allowed the development of an endograft based on the hourglass concept, which was applicable to 85.8% of the patients. The in vitro model of juxtarenal aneurysm evidenced good radiopacity and functionality and permitted adjustments in the new device and technical improvements in the procedures for treating these aneurysms. In addition, the porcine model of juxtarenal aneurysm was successfully created in all six animals using a bovine pericardial patch, and use of the new endograft in three pilot procedures evidenced its feasibility.
CONCLUSIONS:The Hourglass endograft was rendered applicable to treatment of the majority of patients with juxtarenal aneurysms simply by changing its diameter. Moreover, the new in vitro and in vivo models were shown to be effective for assessing both the presented endograft and experiments assessing the endovascular treatment of juxtarenal aneurysms.
Juxtarenal aneurysms (JRAs) are defined as short-necked (<15 mm) infrarenal aortic aneurysms and comprise 15% of all abdominal aortic aneurysms 1. For patients younger than 65 years of age with low surgical risk, open repair is considered the gold-standard treatment, and early mortality ranges from 0% to 8.6%. Complications associated with supra-renal clamping are reported in 3.1% to 6.1% of patients 2, and 0% to 3.8% of all patients require permanent dialysis 3. Endovascular repair (EVAR) is indicated for older patients and for those with severe comorbidities or high surgical risk, and early mortality ranges from 0.8% to 4.1% 4.
Endovascular procedures for the treatment of JRA include a hybrid technique, fenestrated or branched endografts, and the chimney technique 5 and are associated with more early and late postoperative complications, and especially renal insufficiency, endoleaks and thrombosis of the stent 6. Certain authors consider endovascular approaches to be contraindicated for JRA 4, but a recent systematic review 7 comparing open and endovascular repair of JRA showed promising results for EVAR, with significantly lower rates of two-year mortality compared with open repair (16% and 23%, respectively). However, the rates remain higher than those observed in infrarenal aneurysms with a neck length >15 mm (7.8%) 8.
Conventional surgery for JRA remains associated with high rates of morbidity and mortality. However, even for well-selected patients, most fenestrated or branched endografts must be customized to each patient, resulting in very difficult and expensive access to such technology. All of these issues highlight the need for new endovascular devices that are feasible for the treatment of JRA.
Based on this need, the objectives of the present study were as follows: 1) to analyze the angiotomographic parameters of patients with JRA; 2) to adjust a projected endograft model for JRA repair based on the angiotomographic analysis, 3) to create an in vitro model to assess the new endograft model, and 4) to test the new endograft in a newly created porcine model of JRA.
METHODSAngiotomographic parametersAngiotomographies of 49 patients with JRA (neck length <15 mm) treated between January 2009 and October 2013 were selected from the hospital registry, and the parameters below were analyzed using OsiriX image-processing freeware.
Parameters included the aortic diameter of the celiac trunk (CT), superior mesenteric artery (SMA), higher renal artery (HRA) and lower renal artery (LRA); angulations of the CT, SMA, right renal artery (RA), and left RA; longitudinal distances between the CT and the SMA, between the SMA and the HRA, and between the SMA and the LRA; and the length of the neck.
Each patient's angiotomographic parameters were correlated using Pearson's correlation coefficient. After constructing a 95% confidence interval, descriptive measures were analyzed using Student's t test. Statistical observations were used to assess the applicability of the projected endograft.
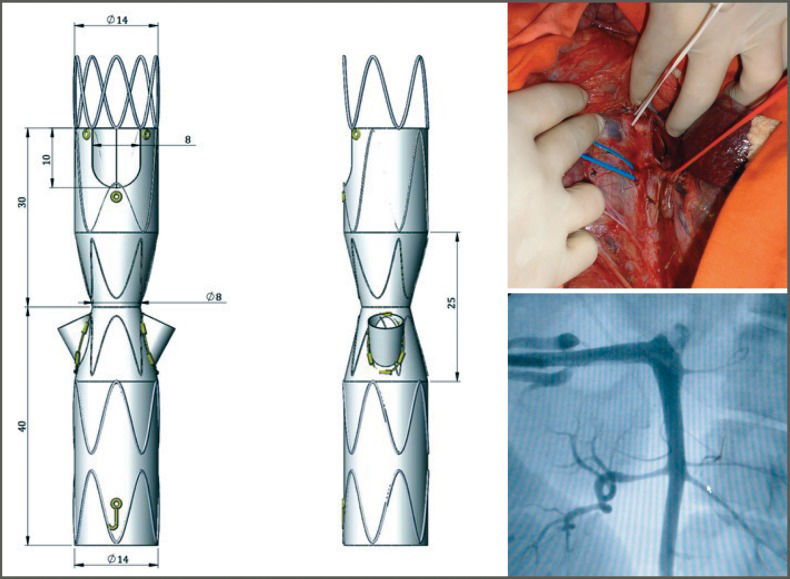
Projected endograftThe Hourglass endograft (Figure 1) was projected in partnership with Braile Biomédica (São Paulo, Brazil) based on several main principles, as follows:
- •
proximal free flow to increase the fixation area using an uncovered stent;
- •
inner branching to gain external area and to allow sufficient area for the apposition of a covered stent, avoiding endoleaks;
- •
a distal area functioning as a longer “neo-neck” of the aneurysm for overlap with a conventional endograft to treat infrarenal aneurysms;
- •
a proximal anterior scallop (in a half-moon shape) to maintain the patency of the SMA;
- •
hourglass-like narrowing (<35%) to create a good distance between the endograft and the aortic wall, allowing easier catheterization of the RAs, even if in different positions in both the anteroposterior and the inferosuperior directions.
An in vitro model of JRA was designed, as shown in Figure 2, and was manufactured using glass.
The projected endograft was tested in the in vitro model while employing a hemodynamic procedure, using the same materials as if treating a human being. After releasing the Hourglass endograft, the covered stents (Viabhan-GORE®) were placed in the RA, and a bifurcated endograft usually used for the treatment of infrarenal aneurysms was placed to complete the testing procedures (Figure 3). The functionality and radiopacity of the model and its adherence to the endograft material were then assessed.
Porcine model of JRAAn experimental model of JRA was created in six domestic white pigs weighing 50 kg to 60 kg (R.G. Grange, Mea Farm, Suzano, São Paulo, SP, Brazil). All pertinent guidelines described by current Brazilian legislation (Decree 6,899/2009) on the use and protection of animals in experimental research were rigorously followed, and this study was approved by the Ethics Board in Scientific Research of the Medical School of São Paulo University (protocol number 431/11).
Three pigs were used in a pilot experiment, and the feasibility of the procedure, the catheterization of the RAs, and the patency and pressure rates were assessed before and after deploying the device. Based on this assessment, the endograft was adapted to the porcine model with an external covered fenestrated segment and was then placed in another three pigs and again assessed (Figure 4).
The animals were submitted to pre-anesthesia with intramuscular ketamine and midazolam. Anesthesia was induced with intravenous thiopental and maintained with continuous inhalation of halothane and intravenous fentanyl. Physiological breathing parameters were maintained with mechanical ventilation. Cardiac frequency, invasive arterial pressure and central venous pressure were continuously assessed until the animals were sacrificed.
RESULTSThe analysis of angiotomographic parameters revealed significant positive correlations among aortic diameter measurements at different locations (range: r=0.71 to r=0.93) and between the longitudinal SMA-HRA and SMA-LRA distances (r=0.59), indicating that variations in such parameters in each patient followed a very homogeneous pattern. No correlation was found for angulations or the other longitudinal distances, and the individual patterns of these variations were heterogeneous (Figure 5). Descriptive measures for all 49 patients are presented in Table 1.
Descriptive measures of the angiotomographic parameters of 49 patients with juxtarenal aneurysm.
| Parameter | Descriptive measures | |||||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | Standard deviation | Median | ||
| Aortic diameters | CT | 1.38 | 3.11 | 2.55 | 0.40 | 2.62 |
| SMA | 1.40 | 3.12 | 2.41 | 0.41 | 2.45 | |
| RMA | 1.41 | 3.01 | 2.34 | 0.44 | 2.32 | |
| RMB | 1.36 | 2.99 | 2.34 | 0.43 | 2.41 | |
| Angulations | CT | 1.72 | 351.28 | 29.64 | 48.34 | 24.11 |
| SMA | 1.10 | 352.24 | 64.08 | 117.42 | 18.48 | |
| Right RA | 266.77 | 321.97 | 296.03 | 12.79 | 292.63 | |
| Left RA | 36.89 | 137.66 | 89.72 | 21.77 | 94.34 | |
| Longitudinal distances | CT-SMA | 0.40 | 2.70 | 1.45 | 0.51 | 1.40 |
| SMA-HRA | 0.17 | 2.20 | 1.15 | 0.46 | 1.20 | |
| SMA-LRA | 0.30 | 2.91 | 1.74 | 0.47 | 1.70 | |
| Length of neck | 0.01 | 1.41 | 0.70 | 0.42 | 0.73 | |
JRA: juxtarenal aneurysm; CT: celiac trunk; SMA: superior mesenteric artery; HRA: higher renal artery; LRA: lower renal artery; RA: renal artery.
According to these findings, the projected endograft was shown to be applicable in 85.8% of cases. The excluded cases were as follows: one patient with only one kidney, three patients presenting a polar artery, one patient with neck angulation >45°, one patient with SMA angulation >35°, and one patient with a longitudinal SMA-HRA distance <0.40 cm.
The in vitro model of JRA was shown to be functional and to have good radiopacity and optimal adherence to the endograft material, allowing adjustments of the projected endograft and technical improvements in the endovascular procedures.
In three procedures simulating the treatment of JRA, with durations of 42, 48 and 49 min, the covered stents were easily catheterized and released in the RA.
A porcine model of JRA was successfully achieved in all six pigs studied. The experimental procedures lasted 2 h 20 min, 1 h 55 min or 2 h 05 min and the necessary contrast volumes were 85 ml, 110 ml and 120 ml, respectively. During deployment of the adapted Hourglass endograft, difficult catheterization of the RA resulted from the posteriorization caused by the aneurysm model. The diameter prevented the release of the distal bifurcated endograft. Thrombosis of one covered stent was observed in the control angiogram, and endoleaks were not observed. Moreover, no difference was observed between the pressure rates measured before and after deploying the endograft. Figure 6 illustrates the results of the experimental procedure.
DISCUSSIONConventional surgical treatment of JRA is a complex procedure associated with more risks than surgical treatment of infrarenal aneurysms, especially considering postoperative renal morbidity. However, the success of endovascular treatment of abdominal aortic aneurysms is dependent on the site of proximal anchorage of the endograft, and approximately 30% of the cases are not eligible for EVAR with the currently available technologies because of unfavorable anatomy 6. Most recommendations refer to a proximal neck length ≥15 mm for adequate proximal anchorage of the endograft and cite proximal necks with a circumferential mural thrombus or with severe angulation >60° as a contraindication 5.
Several endovascular techniques have been developed in an attempt to repair JRA. The hybrid technique combines endograft deployment with occlusion of the entry of visceral arteries previously revascularized with grafts. However, the surgical stages are complex and long, causing significant morbidity and very variable results 9. In the chimney technique, the stent is placed through the external part of the endograft to pass through the vessel and restore the visceral flow, but such presentation of the stent in parallel with the endograft can result in endoleaks and in stenosis or occlusion of the stent 10.
Fenestrated and branched endografts were developed in an attempt to increase the use of endografts by possibly moving the anchorage area proximally to the RA; both types of endografts could be customized based on imaging studies 10 but were expensive and require an extended period of time to be customized and prepared, delaying repair and thus limiting their use 9. In most cases, catheterization of the visceral branches through the orifices or branches represented a critical challenge, requiring considerable time and effort, even for expert surgeons. Such difficulty has been minimized with the use of new endografts developed to be deployed through guide wires, which have previously been used to catheterize the visceral vessels 11. Furthermore, the success of deploying fenestrated and branched endografts is dependent on the planned placement of the orifices or branches because there is wide variation in the anatomy of the visceral arteries emerging at the aorta. Considering the most ventral point of the aorta to be at 12:00, a scallop fixed at 12:00 for the SMA, fenestration for the left RA oscillating between 9:15 and 10:15, and fenestration for the right RA oscillating between 2:15 and 3:15 have already been observed in a set of 439 customized endografts 12. The distance between the origin of the orifice of the RA and the proximal end of the endograft ranged from 19 mm to 28 mm. Although such data were collected while developing customized endografts 12, they also indicate the usefulness of measuring certain parameters in a large number of angiotomographies to characterize the emergence of visceral arteries at the aorta and to thus aid the development of new, more adequate endografts to be used in the treatment of JRA.
The Hourglass endograft presented in the present study was projected without requiring the proximal neck for its fixation. For this purpose, the endograft had a proximal scallop at 12:00 for the SMA and two small inner branches for the RA, with lateral exits for the deployment of covered stents. Considering the variations in the positions of RA emergences, the endograft was hourglass shaped in the small-branch segments to allow angulation of the covered stent that catheterized the RA.
The angiotomographic parameters evaluated in this study were consistent with those reported in the literature. Regarding the angulation of the origin of the visceral arteries, Ferreira et al. 13 reported similar measures when developing 21 branched stent grafts for the repair of thoracoabdominal aneurysms in high-risk patients. The longitudinal CT-SMA and SMA-HRA distances in the current study were also very similar to those reported by Takahashi et al. 14 in a study using 20 cadavers to clarify the positional relationships between the CT, the SMA, and the RA. Using these parameters, we confirmed the applicability of the Hourglass endograft in 85.8% of the 49 patients with JRA who were submitted to angiotomographic assessment.
The in vitro model created specifically to test and adjust the Hourglass endograft seems to be the first reported model of JRA in glass. Several authors have already reported the use of an aortic model in glass in studies regarding staged endovascular treatment for complicated type B dissection 15, flow velocity and turbulence in the transverse aorta of a proximally directed aortic cannula 16, staged proximal deployment of thoracic stent grafts 17, the experimental fluid dynamics of transventricular apical aortic cannulation 18, and the hydrodynamics of aortic cannulas during extracorporeal circulation 19. Berger et al. 20 also proposed a dry glass model of the abdominal aorta and its tributaries with various stenoses, elongations, and tortuosities, named the STRESS machine, to test the endovascular skills of vascular surgeons.
Using a glass model of JRA that was created to test the Hourglass endograft, covered stents were easily catheterized and released in the RA in the current study. The functionality and radiopacity of this glass model and its optimal adherence to the endograft material allowed for certain adjustments to the Hourglass endograft, indicating its usefulness in making future technical improvements in endovascular procedures.
The development of a new endograft requires in vivo assessment. Several techniques for creating experimental aneurysms in vivo have been reported in the literature, including genetic induction, interposition graft use, transluminal dilatation, and infusion of substances such elastase. Patch deployment is the most frequently used model because it is simple to perform, fast and secure 21,22. To date, however, we have not found any published reports regarding models of JRA or thoracoabdominal aneurysms.
The porcine model of JRA presented here was developed to assess the Hourglass endograft and was successfully implemented in six animals, and the feasibility of the endograft was demonstrated (Figure 6). Deployment of the new endograft occurred with certain expected difficulties related to porcine anatomy, but endoleaks were not observed in the imaging studies. Thrombosis of a covered stent was also observed in the control angiogram in one case.
The development of the Hourglass endograft allowed us to create feasible in vitro and in vivo models of JRA. Because the angiotomographic parameter analysis revealed the applicability of this endograft to more than 80% of the patients involved in the study, the next steps include more in vitro testing using a pressurized system and assessment of both its feasibility and potential technical improvements in the procedure using cadavers. After such analysis, a protocol will be developed for use in human beings, affording the opportunity to treat patients with JRA in a faster, cheaper and more secure way.
CONFLICT OF INTERESTThe endografts and covered Viabhan® stents used in the study were donated by Braile-Biomedica® and GORE®, respectively.
AUTHOR CONTRIBUTIONSBelczak SQ and Silva ES participated in the new endograft design and in vitro and in vivo model conception, development and procedures. Botelho Y collected the data. Belczak SQ analyzed the data. Belczak SQ, Lanzaiotti L, Botelho Y, Aun R, Silva ES, Puech-Leão P, and de Luccia N participated in the writing, review and approval of the manuscript.