The standard therapy for patients with high-level spinal cord injury is long-term mechanical ventilation through a tracheostomy. However, in some cases, this approach results in death or disability. The aim of this study is to highlight the anesthetics and perioperative aspects of patients undergoing insertion of a diaphragmatic pacemaker.
METHODS:Five patients with quadriplegia following high cervical traumatic spinal cord injury and ventilator-dependent chronic respiratory failure were implanted with a laparoscopic diaphragmatic pacemaker after preoperative assessments of their phrenic nerve function and diaphragm contractility through transcutaneous nerve stimulation. ClinicalTrials.gov: NCT01385384.
RESULTS:The diaphragmatic pacemaker placement was successful in all of the patients. Two patients presented with capnothorax during the perioperative period, which resolved without consequences. After six months, three patients achieved continuous use of the diaphragm pacing system, and one patient could be removed from mechanical ventilation for more than 4 hours per day.
CONCLUSIONS:The implantation of a diaphragmatic phrenic system is a new and safe technique with potential to improve the quality of life of patients who are dependent on mechanical ventilation because of spinal cord injuries. Appropriate indication and adequate perioperative care are fundamental to achieving better results.
Spinal cord injury (SCI) is a serious condition that mainly affects young adults and often results in death or disability. The critical loss of neurological function below the level of the injury leads to multiple adverse effects, particularly in the respiratory system. Approximately 50% of SCI patients develop quadriplegia, with 4% requiring mechanical ventilation (1). The standard therapy for patients with high-level SCI is long-term mechanical ventilation through tracheostomy. However, this treatment is associated with adverse effects, such as a higher incidence of lung infection and death (2). Furthermore, in USA, the estimated life expectancy for a 20-year-old patient who has an SCI and is dependent on mechanical ventilation decreases from 58.6 to 17.1 years (1).
The concept of phrenic nerve stimulation, or electric ventilation, is not new (3). More recently, a new device that focuses on the phrenic nerve motor point stimulation on the abdominal portion of the diaphragm was developed to allow patients to be weaned from mechanical ventilation (2). The inclusion criteria for diaphragmatic pacemaker implantation are chronic ventilator-dependent high-level SCI, a stimulable diaphragm and preserved phrenic nerves. A successful result depends on the ability of the pacemaker system to provide adequate tidal volume and to ultimately allow weaning from the mechanical ventilation (4),(5).
There are few previous publications concerning the perioperative management of patients undergoing diaphragmatic pacemaker implantation. The aims of this study are to report on five cases of high cervical SCI treated with the laparoscopic insertion of a diaphragmatic pacemaker and to highlight the anesthetic and perioperative aspects of each case.
MATERIALS AND METHODSThe patients in this report were part of a funded pilot project conducted by the thoracic surgery and neurosurgery departments at the Heart Institute (InCor, São Paulo, SP, Brazil) (6). This study was approved by the InCor Ethical Committee (CAPPesq n 0551/10).
Five patients presenting with quadriplegia after high cervical traumatic SCI and ventilator-dependent chronic respiratory failure were scheduled to undergo laparoscopic implantation of the NeuRx® Diaphragm Pacing System (DPS) (Synapse Biomedical, Oberlin, OH, USA).
All of the patients were selected after preoperative assessments of their phrenic nerve function and diaphragm contractility using transcutaneous nerve stimulation. All of the patients were tracheostomized and dependent on mechanical ventilation. The patients’ clinical and demographic data were recorded (Table 1).
Clinical characteristics of the patients who underwent DPS implantation.
| Subject | Sex | Age | Level | MV Time | MV Dependence |
|---|---|---|---|---|---|
| LMFF | F | 26 y | C3 | 9 y | Complete |
| PIS | F | 35 y | C2C3 | 14 y | Complete |
| EFS | M | 27 y | C4 | 1 y | Complete |
| VOS | M | 16 y | C4C5 | 10 m | Partial (with supplemental oxygen) |
| IFLS | F | 40 y | C3 | 6 y | Complete |
MV: mechanical ventilation; y: years; m: months.
Preoperative anesthetic assessments were performed, and all of the patients were pre-medicated with 0.1 mg/kg oral midazolam 30 minutes before arriving in the operating room. The induction of anesthesia was achieved with intravenous 5 μg/kg fentanyl and 2 mg/kg propofol. The bi-spectral index (BIS) was used during anesthesia, and a range of 40–60 was maintained during the procedure to allow for adequate sedation levels. No neuromuscular blockers were given during the procedure. Anesthesia was maintained with 1.5–2% sevoflurane and a mixture of 60% oxygen and nitrogen; 1 µg/kg fentanyl was administered in bolus according to anesthetic criteria. If appropriate hypnosis was not reached, an intravenous infusion of 0.25 µg/kg/min propofol was started. The patients were monitored with electrocardiography, pulse oximetry, capnometry, and non-invasive arterial pressure. All of the patients were ventilated with a tidal volume of 8 ml/kg. The respiratory rate was maintained at 10–16 breaths per minute. A positive end expiratory pressure (PEEP) level of 5 cmH20 was achieved (or higher, as necessary) to reach an oxygen saturation level equal to or higher than 95%.
The surgical technique employed is described in detail in a previous publication (7). A brief summary follows. Laparoscopy was performed using a peritoneal CO2 insufflation pressure between 12 and 15 cm H20. There was no complication related to the trocar insertion. If a patient presented with hypotension (defined as systolic arterial pressure [SAP] lower than 90 mmHg or mean arterial pressure [MAP] lower than 65 mmHg) during peritoneal insufflation, a 500 mL bolus of lactated Ringer’s was administered. If hypotension persisted, 5 mg ephedrine in bolus was administered. None of our five patients presented with dysreflexia or vasopressor/inotropic support requirements.
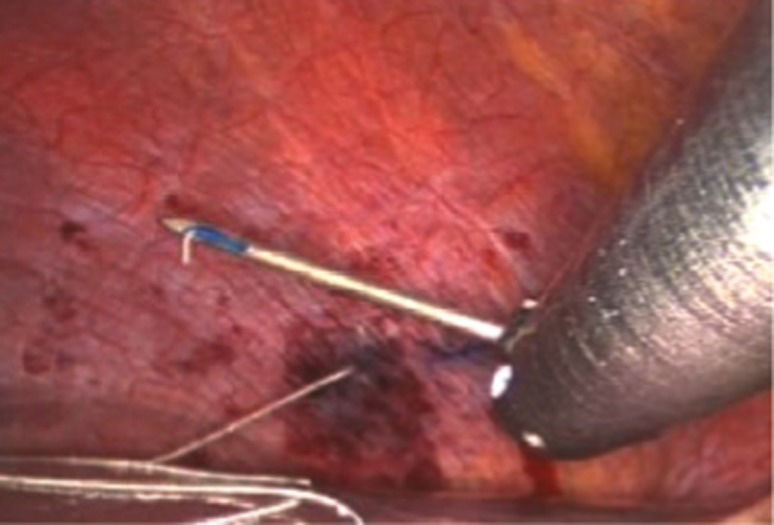
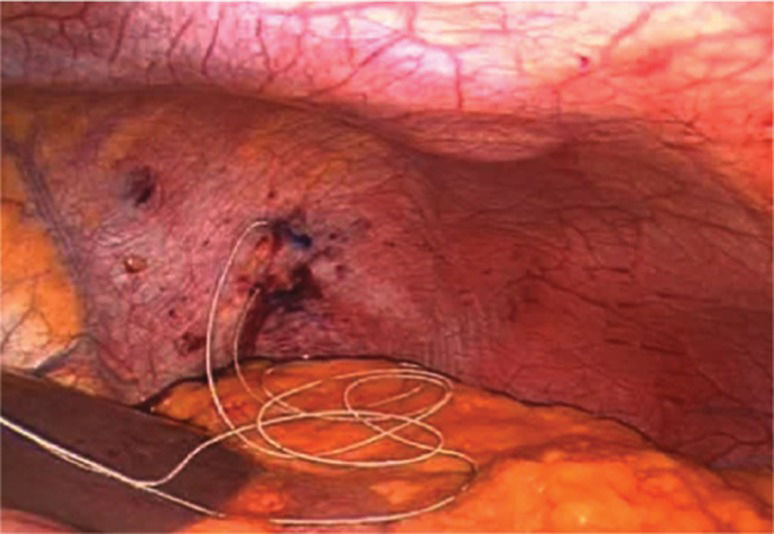
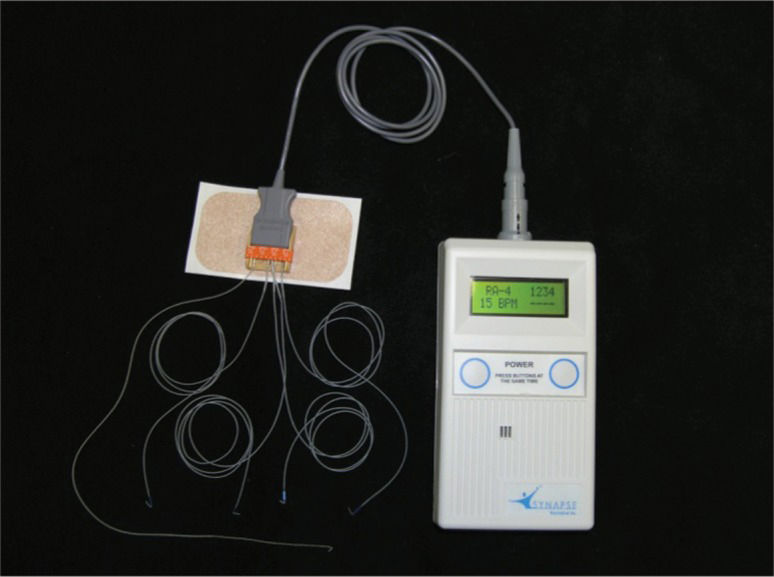
The initial phase included mapping each hemidiaphragm by systematically stimulating it under direct vision to determine two sites for the permanent pacing of the phrenic nerves (Figure 1). These permanent electrodes were inserted into the diaphragm: one centrally and one posteriorly (Figure 2). A clinical station (Synapse Biomedical, Oberlin, OH, USA) was used to establish the parameters of the pacing device (Figure 3).
After implantation, the DPS was tested by switching the ventilation mode of the anesthesia machine to the spontaneous mode. An electrocardiogram strip was also recorded to ensure that the left-sided pacing did not affect the cardiac rhythm (8).
RESULTSThe procedures were performed successfully. One patient had an uncuffed tracheostomy tube and developed bilateral capnothorax during surgery, which resulted in higher ventilatory pressure. The condition was rapidly diagnosed by evaluating the pulmonary pressure and was immediately resolved after deflating the abdominal insufflation.
In another patient with a raised hemidiaphragm, a Valsalva maneuver was performed to produce downward force to help insert the permanent electrode. The Valsalva maneuver produced hypotension that resolved after releasing the maneuver.
Another patient presented with capnothorax on a post-operative chest X-ray, and the decision was made to perform pleural drainage with a pigtail catheter. After the procedure, all of the patients recovered from anesthesia and were returned to their previous respiratory support. The patients were then transferred to the intensive care unit. On the second postoperative day, the patients began conditioning training of the diaphragm muscle through the intermittent use of the DPS for progressively longer time periods (Figure 4).
Six months following the procedures, three patients achieved continuous DPS use for 24 hours per day. A patient who had relied on mechanical ventilation for 14 years achieved respiration for 6 hours each day with the pacemaker. However, diaphragmatic stimulation was discontinued in this patient after the onset of uncontrolled neuropathic pain. The fifth patient was unable to sustain ventilation with the DPS.
DISCUSSIONOur study illustrates the perioperative management of patients with severe SCI and chronic respiratory failure who underwent insertion of DPS. This technique allows patients to wean from mechanical ventilation.
Mechanical ventilation-related adverse events and safety issues have been studied extensively in experimental (9) and clinical trials (10),(11) that emphasize the importance of early extubation. The laparoscopic approach to DPS implantation in our SCI patients was a safe and efficient procedure with no severe complications.
Electric stimulation of the phrenic nerve for diaphragm pacing in patients with SCI has been well documented beginning with the pioneering work of Glenn (3). A more recent study focused on the phrenic nerve motor point in the diaphragm (12) and has created innovative applications of diaphragm pacing in other populations, such as patients with congenital central hypoventilation syndrome (13), amyotrophic lateral sclerosis (14),(15), and acute respiratory failure (16).
Few studies are available on the perioperative management of patients with SCI. Although pre-medication can be used, there is concern regarding the higher sensitivity to medication in patients with SCI compared to the general population, and a lower dose is thus recommended.
The specific challenges of this technique include performing the procedure without muscle relaxants, avoiding hemodynamic instability secondary to pneumoperitoneum and autonomic disorders that may be worsened by the procedure (17). Neuromuscular blocking agents must be avoided because they could interfere with the intraoperative mapping of the diaphragm. Nevertheless, in our study, all of the patients already had tracheostomies placed, and the absence of neuromuscular blockers did not represent an obstacle because tracheal intubation was unnecessary.
After venous induction, patients can present with severe hypotension because of the absence of a sympathetic reflex and relative hypovolemia. Before the induction of general anesthesia, 500 to 1000 mL of crystalloid solution is recommended. Patients with SCI also present a higher risk of hypothermia; therefore, body temperature must be monitored.
The phenomenon of autonomic dysreflexia is rare but is a severe and life-threatening complication that can occur during invasive surgical procedures in patients with spinal cord injury. Autonomic dysreflexia is characterized by disordered autonomic responses to certain stimuli below the level of the lesion (18). Adequate fluid management, careful perioperative management, and the proper depth of anesthesia can successfully control autonomic dysreflexia. We used BIS to monitor our patients to ensure the ideal anesthesia depth and minimize the risk of autonomic dysreflexia.
Although rare, the artificial pneumoperitoneum created using CO2 can cause pneumothorax/capnothorax, a known complication of laparoscopic surgery. CO2, a highly diffusible gas, can infiltrate into the pleural space through congenital defects of the diaphragm or through parietal pleura injuries that are caused by surgical manipulations. The anesthesiologist must consider the possibility of capnothorax when the patient presents with a sudden reduction in lung compliance, an elevation in peak respiratory pressure and end-tidal carbon dioxide (ETCO2). Hypotension and hypoxemia are uncommon events in this scenario. The resolution of the capnothorax can be accelerated by using PEEP and hyperventilation (19).
The most common complication that occurred in our patients was capnothorax, which was observed in two cases. These events were most likely related to the electrode implantation procedure. Note that the incidence of capnothorax observed during the implantation of the NeuRx DPS in patients with amyotrophic lateral sclerosis (ALS) was lower than for the SCI patients. In the ALS cases, capnothorax occurred in 16 of 86 (19%) patients and in the SCI cases, capnothorax occurred in 21 of 50 (42%) patients (20).
In another case series of six patients, Onders et al. (21) reported the possible complications of wound infection and intermittent aspiration. In our study, we observed incidence of infection.
The efficacy of DPS has been suggested by Alshekhlee et al., (22) who described a series of 26 patients who received successful DPS implantations. In that study, 96% of the patients were able to use the DPS, and 54% achieved full-time pacing a median of 142 days following the implantation.
The use of a diaphragm pacing system has changed the medical outcome of SCI and chronic respiratory failure patients who have long-term dependence on mechanical ventilation (23). The improvement in tidal volumes and vital capacity translates into a lower dependence on mechanical ventilation and an increased quality of life. In addition, this implantation technique is related to lower morbidity compared to the traditional phrenic nerve pacing. This improvement is due to the laparoscopic placement of the DPS electrodes, which avoids bilateral thoracic access and the potential risk of phrenic nerve damage (24).
Although the SCI patients presenting with chronic respiratory failure and dependence on mechanical ventilation represent a high-risk group for perioperative complications, the successful implantation of a DPS was achieved in all of the patients in our study.
Our results reinforce the premise that the laparoscopic implantation of a DPS can be safely accomplished if certain principles are followed: a) before surgery, the patients must be evaluated regarding whether the diaphragm is stimulable and to determine that the phrenic nerves are intact; b) optimal anesthetic management includes adequate premedication and avoiding over-sedation by adequate BIS monitoring to allow early recovery and weaning from ventilation; c) avoidance of neuromuscular blockers; d) infection surveillance; and e) adequate post-operative ICU care.
In conclusion, the implantation of a DPS is a safe and efficient procedure with the potential to improve the quality of life of patients who are dependent on mechanical ventilation as a result of SCI. Adequate perioperative care is essential to ensure the best results. Further experimental trials are needed to assess the impact of the DPS technique in these patients.
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – 2010/50785-6). A donation was also received from Synapse Biomedical International.
No potential conflict of interest was reported.
Tedde ML conceived the study, collected the data, participated in the analysis of the samples and drafted the manuscript. Vasconcelos-Filho P, Hajjar LA, Flora GF, Okumura EM and Galas FR collected the data, participated in the analysis of the samples and drafted the manuscript. Almeida JP, Osawa EA and Fukushima JT participated in the analysis of the samples and drafted the manuscript. Teixeira MJ, Jatene FD and Auler Jr JO drafted and approved the manuscript’s final version.