This study aimed to evaluate long-term weight loss in overweight hypertensive patients receiving dietary counseling.
METHODS: Longitudinal study included overweight hypertensive patients who had an initial individual consultation with a nutritionist between January 2002 and December 2005 and were followed for four years in a hypertension clinic. Patients who had at least four consultations during the follow-up period were included in the dietary counseling group. Those who scheduled their first consultation but missed that appointment or had fewer than four consultations during the follow-up period were allocated to the control group. Target Energy intake was calculated at 20–25 kcal/kg actual body weight/day.
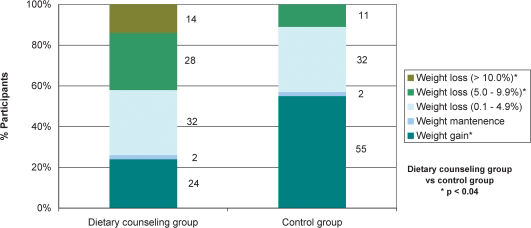
RESULTS:The study included 102 patients aged 55±1 years old (58 in the dietary counseling group). As compared with the control group, patients in the dietary counseling group showed a significantly greater reduction in body weight (-3.6±0.8 vs. 0.8±0.7 kg), which remained significant after controlling for age, gender, baseline body mass index, and the use of different antihypertensive and antidiabetic drugs. Weight loss between 5.0% and 9.9% was observed in a significantly higher percentage of patients in the dietary counseling group (28% vs. 11%). A weight loss of at least 10% was only observed in dietary counseling group patients, who had a significantly lower odds ratio for increasing the number and/or dosage of antihypertensive agents, even after controlling for age, gender, and baseline body mass index.
CONCLUSIONS:Dietary counseling may be associated with long-term weight loss in overweight hypertensive patients.
Obesity is an increasingly prevalent condition throughout the world.1 Body mass index (BMI) is directly associated with high blood pressure and the prevalence of hypertension.2–5 Even a modest adult weight gain substantially increases the risk for hypertension while weight loss reduces blood pressure.6 Overweight and obese hypertensive patients are advised to lose weight to ideally reach a BMI lower than 25 kg/m2.2,7–8 However, decreases in blood pressure occur before and/or without reaching this desired body weight. Weight losses as little as 5.1 kg can decrease systolic and diastolic blood pressures by 4.4 mmHg and 3.6 mmHg, respectively.9
Dietary intervention is an important aspect in the treatment of excess body weight.10 A clinically significant weight loss can be obtained with relatively short-term (i.e., six months to one year) dietary interventions based on energy restriction, regardless of the macronutrient emphasized.11–13 However, obesity is a chronic disease, and weight regain is a disappointingly common problem. Consequently, a long-term approach to treatment is needed.10–15 The effect of dietary counseling on long-term weight change is not clear. The literature on the long-term follow-up of dietary treatment for patients with obesity indicates an overall median success rate of 15%.16 In a recent meta-analysis of randomized controlled trials, nutritional interventions produced a mean 6% weight loss after one year. Approximately half of the initial weight loss was regained after three years.13
Despite evidence that hypertensive patients with excess body weight should lose weight and that lifestyle modifications are essential to treat obesity, specific data on the effect of dietary counseling on the long-term weight loss of hypertensive individuals are scarce. Therefore, this study aimed to evaluate long-term weight loss in overweight hypertensive patients receiving dietary counseling. A secondary purpose was to investigate whether dietary counseling is related to modifications in the metabolic profile, blood pressure levels, and the use of antihypertensive, antidiabetic, and lipid-lowering drugs.
METHODS AND MATERIALSThis was a longitudinal study carried out with overweight and obese hypertensive outpatients from the Hypertension Clinic (CLINEX) of the Rio de Janeiro State University. The Hypertension Clinic is a Brazilian referral center for the multidisciplinary care of hypertensive patients, who are referred by their physicians for individual dietary counseling with nutritionists. Regular dietary counseling, however, depends on the patient's willpower. Patients can schedule dietary counseling at any time during their treatment at the Hypertension Clinic.
The inclusion criteria were as follows: hypertensive patients who had their first individual consultation with a nutritionist between January 2002 and December 2005 and were followed for four years at the Hypertension Clinic; between 30 and 80 years old in age; and BMI of at least 25 kg/m2 (adult) or 27 kg/m2 (elderly).
The exclusion criteria were as follows: current use of medications for weight loss; high level of physical activity; and recent (i.e., within the previous six months) changes in dietary intake, body weight (≥3 kg), or the intensity or frequency of physical exercise. In addition, patients with any of the following were also excluded from the study: eating disorders, major depression, a medical history of drug addiction, hypothyroidism, insulin-dependent diabetes, diseases severely affecting the gastrointestinal and renal systems, and a coronary event or stroke in the three months preceding the selection. Pregnant or lactating women were also excluded.
One hundred forty-eight individuals aged 36 to 78 years old, without gender distinction, were enrolled. Dietary counseling was offered every month during the first three months of treatment and every three months thereafter. The nutritionist provided face-to-face counseling that was individually tailored to each patient. Each counseling session lasted 30 to 60 minutes, during which nutritionists strongly recommended that patients follow an energy-restricted diet and increase their physical activity level, primarily through light- to moderate-intensity leisure activities such as walking. The goal of weight loss was to achieve and maintain a 5% to 10% body weight reduction. All participants consumed self-selected foods. Neither vitamins nor other nutritional supplements were prescribed.
Energy intake was calculated to provide 20 to 25 kcal/kg of actual body weight/day with ~ 15% to 20% of calories from protein, 25% to 30% from fat, and 50% to 60% from carbohydrate. Patients were advised to limit their consumption of energy-dense snacks. Sodium intake was restricted to 2400 mg/day. All patients were also instructed about the dietary approaches to stop hypertension (DASH) eating plan, which aims to increase the consumption of fruits, vegetables, and low-fat dairy products and to reduce saturated and total fat intake. Specific dietary counseling was provided to patients with type 2 diabetes and/or dyslipidemia according to the recommendations of the American Diabetes Association17 and the National Cholesterol Education Program.18 Strategies for continued success were developed and reinforced at each visit.
Patients were considered hypertensive if their systolic and/or diastolic blood pressure levels were at least 140 and 90 mmHg, respectively, or if they were on antihypertensive therapy.7 Diabetes mellitus was diagnosed when fasting glucose was at least 126 mg/dL on two different days or when patients were using insulin or an oral antidiabetic medication for at least eight weeks.19 The diagnosis of dyslipidemia followed the Adult Treatment Panel III criteria18 (total cholesterol ≥200 mg/dL, low-density lipoprotein cholesterol ≥130 mg/dL, or triacylglycerols ≥150 mg/dL) or was determined by the use of lipid-lowering drugs.
Blood samples were collected after a 12-hour fasting period. All biochemical analysis was performed at the clinical laboratory of the Pedro Ernesto University Hospital. Biochemical evaluation examined glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triacylglycerols. Fasting plasma glucose was determined by the glucose oxidase method. The serum lipid profile, including total cholesterol, triacylglycerols, and high-density lipoprotein cholesterol, was assessed using an automated analyzer. If triacylglycerol was lower than 400 mg/dL, low-density lipoprotein cholesterol was calculated using the Friedewald formula.20 Uric acid was measured with an automated technique. Serum urea nitrogen and plasma creatinine were assessed using a kinetic method.
Blood pressure was determined with the auscultatory method in the non-dominant arm after a resting period of at least 10 minutes in the sitting position. A standard mercury sphygmomanometer was used with an appropriate cuff size for each patient. The first value was discarded, and the mean of at least three readings was analyzed.
Height and weight were measured according to standardized procedures; the former was measured with a stadiometer that was accurate within ±0.5 cm while the latter was measured with a calibrated scale that was accurate within ±0.1 kg (Filizola S.A., São Paulo, SP, Brazil) when patients wore light clothing and no shoes. BMI was calculated with the standard formula (kilograms/meters squared).
The study protocol was approved by the committee on ethics of the Pedro Ernesto University Hospital.
Statistical analysisContinuous variables were expressed as means ± standard errors. To test the possible relation of dietary counseling with changes in body weight, biochemical variables and drugs used, patients were stratified into two groups according to adhesion to the dietary counseling provided by nutritionists. Patients who had at least four consultations during the four-year follow-up period of the study were included in the dietary counseling group (DCG). Hypertensive patients who scheduled their first consultation with the dietitian but missed that appointment or had fewer than four consultations during the following four years were allocated to the control group (CG). Continuous variables were compared between the two groups using the Student's t test, and multiple linear regression analysis was used to adjust for factors that could interfere with weight loss (i.e., age, gender, baseline BMI, and the use of antihypertensive and antidiabetic drugs). To evaluate differences in body weight changes between the groups during the study at months 0, 6, 12, 24, 36, and 48, repeated measures ANOVA was applied. The chi-square test was used for comparisons between groups of gender distribuition, prevalence of diabetes and dyslipidemia, and use of drugs. Associations between dietary counseling and changes in the use of different drugs were assessed through multiple logistic regression analysis. As we aimed to evaluate the association between dietary counseling per se and changes in the use of drugs in an observational study, we adjusted our results for factors that could interfere with drug use, specifically gender, age, baseline BMI, and weight loss during the study period. Considering the means and standard deviations of weight loss in both groups during the study period and given a desired power of 0.8, we found that the minimum sample size necessary in each group was 22 participants. We also tested our study power in light of the means and standard deviations of weight loss in both groups during the study period, which was 1.00. Stata 10 (Stata Corp., College Station, TX, USA) was used for statistical analysis. A p-value <0.05 was considered statistically significant.
RESULTSOf the 148 patients who scheduled their first consultation with the dietitian between January 2002 and December 2005 and were eligible for the study, 46 did not complete four years of follow-up in the Hypertension Clinic and were excluded from the study. Therefore, 102 patients (44 men and 58 women) were included in the final analyses. The patients' mean age was 55.1±0.9 years, and their mean BMI was 32.2±0.5 kg/m2. On average, participants included in the study had three to four consultations/year with physicians, and two to three consultations/year with nutritionists. After stratifying the patients according to their dietary counseling (i.e., DCG and CG), both groups were comparable in terms of several demographic, clinical, and biochemical characteristics at the beginning of follow-up (Table 1).
Baseline characteristics of participants according to dietary counseling.
| Characteristics | Dietary counseling group (n = 58) | Control group (n = 44) | p |
|---|---|---|---|
| Age (years) | 54.93±1.20 | 55.35±1.47 | 0.82 |
| Gender (women/men) | 28 (48%) /30 (52%) | 30 (68%) /14 (32%) | 0.05 |
| Body weight (kg) | 86.82±1.93 | 83.12±1.98 | 0.19 |
| Body mass index (kg/m2) | 32.42±0.63 | 31.87±0.71 | 0.57 |
| Glucose (mg/dL) | 120.73±5.53 | 119.07±6.15 | 0.84 |
| Urea (mg/dL) | 34.03 ± 1.79 | 33.46±2.24 | 0.11 |
| Creatinine (mg/dL) | 0.92±0.05 | 0.91±0.07 | 0.64 |
| Uric acid (mg/dL) | 6.00±0.36 | 5.70±0.44 | 0.60 |
| Total cholesterol (mg/dL) | 219.04±6.51 | 230.26 ± 8.72 | 0.37 |
| HDL cholesterol (mg/dL) | 45.88±1.88 | 46.48±2.30 | 0.84 |
| LDL cholesterol (mg/dL) | 137.84±8.01 | 149.08±10.43 | 0.39 |
| Triglycerides (mg/dL) | 190.07±19.58 | 183.85 ± 20.62 | 0.83 |
| Systolic blood pressure (mmHg) | 140.94±2.33 | 141.75±2.93 | 0.83 |
| Diastolic blood pressure (mmHg) | 88.05±1.40 | 87.95±1.65 | 0.96 |
| Mean blood pressure (mmHg) | 105.68±1.53 | 105.89±1.92 | 0.93 |
| Heart rate (bpm) | 72.68±1.51 | 71.92±1.75 | 0.74 |
| Type 2 diabetes (n; %) | 16 (28%) | 14 (32%) | 0.64 |
| Dyslipidemia (n; %) | 47 (81%) | 35 (80%) | 0.57 |
| Use of antidiabetic drugs (n; %) | 10 (17%) | 10 (23%) | 0.55 |
| Use of lipid-lowering drugs (n; %) | 12 (21%) | 9 (20%) | 0.91 |
| Number of antihypertensive agents | 2.25±0.13 | 2.25±0.13 | 1.00 |
Values are expressed as mean ± standard error or percentage.
HDL = high-density lipoprotein, LDL = low-density lipoprotein.
Patients in the DCG showed a significantly greater reduction in body weight during the study period as those in the CG (Figure 1). The difference in weight loss between the two groups remained significant after controlling for age, gender, baseline BMI, and the use of antihypertensive and antidiabetic drugs that could interfere with body weight (p<0.001).
In the DCG, most weight loss occurred in the first six months. At six months, the change from the baseline body weight was significant: -2.80±0.47 kg (-3.2%), p<0.0001. At twelve months of follow-up, this group continued losing weight, and the change compared with baseline was -3.50±0.69 kg (-4.0%). At 24 months of follow-up, patients were still losing weight: -3.83±0.80 kg (-4.3%). After 24 months, patients in the DCG started to regain weight. However, body weight compared with the baseline remained significant lower (p<0.0001) at 36 months [-3.61±0.76 kg (-4.1%)] and 48 months [-3.64±0.79 kg (-3.9%)].
Figure 2 shows that at the end of follow-up, a significantly lower percentage of patients in the DCG gained weight as compared with those in the CG. In both groups, only 2% of patients maintained their weight while 32% lost between 0.1% and 4.9% of their initial body weight. A weight loss of 5.0% - 9.9% (as compared with baseline body weight) was observed in a significantly higher percentage of DCG patients. Weight losses of at least 10% were seen only in DCG patients.
The metabolic variables and blood pressure values during follow-up were compared between groups, and no significant change was observed in the CG. However, in the DCG, significant reductions in glucose, total cholesterol, and triglycerides were observed (Table 2).
Metabolic variables and blood pressure levels at baseline (Month 0) and at the end of follow-up (Month 48) in the two groups.
| Dietary counseling group(n = 58) | Control group(n = 44) | p** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Month 0 | Month 48 | Δ | p* | Month 0 | Month 48 | Δ | p* | ||
| Glucose (mg/dL) | 120.73±5.53 | 108.48±4.15 | -12.25±4.15 | 0.009 | 119.07±6.15 | 117.67±7.28 | -1.40±7.30 | 0.85 | 0.18 |
| Urea (mg/dL) | 34.03±1.79 | 35.16±2.16 | 1.13±1.71 | 0.51 | 33.46±2.24 | 36.80±2.24 | 3.36±1.94 | 0.10 | 0.41 |
| Creatinine (mg/dL) | 0.92±0.05 | 0.98±0.05 | 0.06±0.03 | 0.05 | 0.91±0.07 | 0.94±0.05 | 0.04±0.05 | 0.46 | 0.68 |
| Uric acid (mg/dL) | 6.00±0.36 | 5.63±0.35 | -0.36±0.25 | 0.17 | 5.70±0.41 | 5.73±0.41 | 0.03±0.31 | 0.92 | 0.33 |
| Total cholesterol (mg/dL) | 219.04±6.51 | 202.31±5.60 | -16.73±5.40 | 0.003 | 230.26±8.72 | 221.00±8.49 | -9.26±8.34 | 0.28 | 0.43 |
| HDL-cholesterol (mg/dL) | 45.88±1.88 | 43.71±1.56 | -2.17±1.36 | 0.12 | 46.48±2.30 | 46.13±2.28 | -0.34±1.92 | 0.86 | 0.43 |
| LDL-cholesterol (mg/dL) | 137.84±8.01 | 133.50±7.10 | -4.33±8.60 | 0.62 | 149.08±10.43 | 147.79±8.74 | -1.29±10.13 | 0.90 | 0.83 |
| Triglycerides (mg/dL) | 190.07±19.58 | 163.15±13.61 | -26.92±12.7 | 0.04 | 183.85±20.62 | 157.85±17.11 | -26.00±19.1 | 0.18 | 0.97 |
| Systolic BP (mmHg) | 140.95±2.33 | 138.58±2.69 | -2.36±2.85 | 0.41 | 141.75±2.93 | 143.02±2.72 | 1.27±3.57 | 0.72 | 0.42 |
| Diastolic BP (mmHg) | 88.05±1.40 | 85.23±1.71 | -2.81±1.67 | 0.10 | 87.95±1.65 | 88.41±1.81 | 0.45±2.09 | 0.83 | 0.22 |
| Mean BP (mmHg) | 105.68±1.53 | 103.02±1.86 | -2.67±1.92 | 0.17 | 105.89±1.92 | 106.61±1.96 | 0.73±2.44 | 0.77 | 0.27 |
| Heart rate (bpm) | 72.68±1.51 | 71.45±1.59 | -1,24±1.92 | 0.52 | 71.92±1.75 | 72.10±1.48 | 0.18±2.19 | 0.93 | 0.63 |
Data are expressed as mean ± SEM.
HDL = high-density lipoprotein, LDL = low-density lipoprotein, BP = blood pressure, Δ = Month 48 – Month 0.
Patients in the DCG had a significantly lower odds ratio for increasing the number and/or dosage of antihypertensive agents, even after controlling for age, gender and baseline BMI. However, after adjusting for weight loss, the odds ratio was no longer significantly lower in the DCG (Table 3).
Odds ratios (95% CIs) for the changes in the use of antihypertensive, antidiabetic, and lipid-lowering drugs during the study period according to dietary counseling.
| Control group (n = 44) | Dietary counseling group (n = 58) | p | |
|---|---|---|---|
| Increased the number and/or dosage of antihypertensive agents | |||
| N of cases (%) | 28 (64%) | 21 (36%) | - |
| Odds ratio (95% CI) | 1.00 | 0.32 (0.14 - 0.74) | 0.008 |
| Multivariate-adjusted* | 1.00 | 0.38 (0.16 - 0.90) | 0.03 |
| Multivariate-adjusted** | 1.00 | 0.36 (0.15 - 0.87) | 0.02 |
| Multivariate-adjusted*** | 1.00 | 0.48 (0.19 – 1.22) | 0.12 |
| Began to use or increased the number and/or dosage of antidiabetic drugs | |||
| N of cases (%) | 11 (25%) | 7 (12%) | - |
| Odds ratio (95% CI) | 1.00 | 0.41 (0.14 – 1.17) | 0.09 |
| Multivariate-adjusted* | 1.00 | 0.31 (0.10 - 0.97) | 0.04 |
| Multivariate-adjusted** | 1.00 | 0.30 (0.09 - 0.95) | 0.04 |
| Multivariate-adjusted*** | 1.00 | 0.36 (0.11 – 1.22) | 0.10 |
| Began to use or increased the dosage of lipid-lowering drugs | |||
| N of cases (%) | 7 (16%) | 4 (7%) | - |
| Odds ratio (95% CI) | 1.00 | 0.40 (0.11 - 1.47) | 0.17 |
| Multivariate-adjusted* | 1.00 | 0.36 (0.09 - 1.36) | 0.13 |
| Multivariate-adjusted** | 1.00 | 0.36 (0.09 - 1.39) | 0.14 |
| Multivariate-adjusted*** | 1.00 | 0.34 (0.08 - 1.47) | 0.15 |
The DCG also had a lower odds ratio for beginning the use or increasing the number and/or dosage of antidiabetic drugs after controlling for age, gender, and baseline BMI (Table 3).
DISCUSSIONIn the present study, using a sample of hypertensive patients with excess body weight, followed up for four years and stratified into two groups according to dietary counseling, the main finding was the significantly greater weight loss observed in the DCG. Moreover, a significantly lower percentage of individuals who increased the number of antihypertensive agents and/or their dosage during follow-up was observed in the DCG as compared with those in the CG.
At six months of follow-up, patients in the DCG had lost an average of 2.8 kg, which is lower than the results seen in other trials employing comprehensive lifestyle interventions in hypertensive or prehypertensive individuals.21–23 In the intensive intervention phase (Phase I) of the Weight-Loss Maintenance Trial,21 after six months of intervention, overweight and obese adults on medications for hypertension and/or dyslipidemia showed a mean weight loss of 5.8 kg. Cakir and Pinar (2006)22 conducted a six-month randomized controlled trial on comprehensive lifestyle modification in hypertensive patients. The intervention group showed a mean weight loss of 3.8 kg. This difference in weight loss at six months of follow-up between other studies and ours may be explained by the fact that in other studies, a higher number of weight-loss counseling sessions were offered. For example, in the intensive intervention phase (Phase I) of the Weight-Loss Maintenance Trial,21 20 group weight-loss counseling sessions were offered over six months while in our study, three individual weight-loss counseling sessions were offered during the first six months.
At 12 and 24 months of follow-up, the mean weight loss in the DCG was 3.5 kg and 3.8 kg, respectively, similar to the results of other studies.24–25 In a multicenter randomized trial24 involving 810 adults with prehypertension or stage 1 hypertension, patients were assigned to one of the following three groups: an advice-only comparison group (i.e., “advice only”); an intervention group following an established guideline-recommended lifestyle (i.e., “established”); or an intervention group following the established guideline-recommended lifestyle in addition to the DASH eating plan (i.e., “established plus DASH”). As compared with the “advice only” group, both behavioral interventions significantly reduced body weight after 18 months of follow-up. Weight loss was 3.8 kg in the “established” group and 4.3 kg in the “established plus DASH” group.24 In the Nonpharmacologic Interventions in the Elderly (TONE) Trial, the average reduction in weight for the participants assigned to weight loss intervention was approximately 3.8, 3.6, and 3.9 kg at the 9-, 18- and 30-month follow-up visits, respectively.25
Little is known about the efficacy of long-term lifestyle interventions for weight loss in hypertensive individuals. In the Treatment of Mild Hypertension Study (TOMHS), the weight loss with lifestyle intervention was 4.5 kg (5.6%) at one year, 3.6 kg (4.5%) at two years, 3.2 kg (4.0%) at three years, and 2.3 kg (3.0%) at four years.26 The weight loss obtained in that study at four years of follow-up is lower than that observed in DCG patients in our study for the same period (3.6 kg; 3.9%).
In our study, the weight loss in the DCG during the first six months was lower than that observed in other studies21–23 and, at one year, lower than that reported in the TOMHS Study.26 However, at the end of the follow-up (four years), the weight loss in our study was greater than that observed in the TOMHS Study.26 This is an important finding because, as mentioned in the introduction, the greatest difficulty to treat obesity is to maintain weight loss. The greater weight loss at four years of follow-up in the present study's DCG may have many explanations. One explanation may be the lower weight loss of our patients in the beginning of the follow-up, because a moderate weight loss, as compared with a more substantial one, is more likely to be maintained over a longer period of time.27 Nevertheless, the weight loss observed during the first year in the TOMHS Study is in accordance with the recommendations for lifestyle interventions of different guidelines.8,10
In the present study, a significantly lower percentage of individuals in the DCG as compared with CG patients increased the number and/or dosage of antihypertensive and antidiabetic agents (after adjusting for age and gender) during follow-up. These findings can be explained by the greater weight loss observed in the DCG; after adjusting for that variable, the difference between groups was no longer significant. More than 40% of patients in the DCG lost ≥5% of their initial body weight while only 11% of patients in the CG reached that level of weight loss. A modest weight loss (∼5% to 10% of the initial weight) contributes to important health benefits, improving many of the obesity-related risk factors for cardiovascular disease, such as insulin resistance, dyslipidemia, hypertension, and inflammation.8,10,28–29 In patients on antihypertensive medication, a modest weight loss improves blood pressure control, reducing or even eliminating the need for antihypertensive medication.25,30–33 The blood pressure–lowering effect of weight loss is most likely a result of an improvement in insulin sensitivity and a decrease in the sympathetic nervous system activity, and it occurs independently of salt restriction.27
A recent review of 18 trials with a wide variation in follow-up durations (i.e., two weeks to three years) has found that weight-reducing diets for overweight hypertensive individuals can induce a modest 3% to 9% weight loss and may decrease the dosage requirements of those on antihypertensive medications.32
Although weight loss can be the most important reason for the lower percentage of patients in the DCG who increased the number and/or dosage of antihypertensive agents, DCG patients were also instructed to modify their ingestion of different dietary components that affect blood pressure, such as sodium, potassium, calcium, magnesium, and alcohol, and they were encouraged to follow the DASH eating plan.2,7,34 Gusmão et al. observed that a special care program, including multidisciplinary activities, increases blood pressure control.35
In addition, only the DCG patients had a significant decrease in glucose, total cholesterol, and triglycerides during the study, which can be explained by their greater weight loss and the specific dietary counseling for the treatment of hyperglycemia and dyslipidemia that they received.
Our study has several limitations, the greatest being that it was not a randomized trial. Patients were allocated in both groups according to their willpower. Most likely, patients in the DCG were more interested in losing weight than those in the CG. However, in randomized trials, only individuals willing to undergo the intervention are included in the study. Although our intervention was not as intensive as those of other trials, we aimed to evaluate weight loss in patients receiving a feasible dietary treatment currently offered to our patients, rather than a more intensive intervention that would not be continued after the end of the study. Another important limitation is that this was a single-center study.
CONCLUSIONSIn conclusion, our study suggests that dietary counseling could be associated with long-term weight loss in overweight hypertensive patients.
To FAPERJ for financial support. To Debora Cristina Torres Valença, Maria de Lourdes Guimarães Rodrigues, Luciene da Silva Araújo, Marcella Guedes, and Carolina Lima for their participation in data collection.
No potential conflict of interest was reported.