Last update: January 2023
More infoTo compare demographic/clinical/laboratory/treatments and outcomes among children and adolescents with laboratory-confirmed coronavirus disease 2019 (COVID-19).
METHODS:This was a cross-sectional study that included patients diagnosed with pediatric COVID-19 (aged <18 years) between April 11, 2020 and April 22, 2021. During this period, 102/5,951 (1.7%) of all admissions occurred in neonates, children, and adolescents. Furthermore, 3,962 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection samples were processed in patients aged <18 years, and laboratory-confirmed COVID-19 occurred in 155 (4%) inpatients and outpatients. Six/155 pediatric patients were excluded from the study. Therefore, the final group included 149 children and adolescents (n=97 inpatients and 52 outpatients) with positive SARS-CoV-2 results.
RESULTS:The frequencies of sore throat, anosmia, dysgeusia, headache, myalgia, nausea, lymphopenia, pre-existing chronic conditions, immunosuppressive conditions, and autoimmune diseases were significantly reduced in children and adolescents (p<0.05). Likewise, the frequencies of enoxaparin use (p=0.037), current immunosuppressant use (p=0.008), vasoactive agents (p=0.045), arterial hypotension (p<0.001), and shock (p=0.024) were significantly lower in children than in adolescents. Logistic regression analysis showed that adolescents with laboratory-confirmed COVID-19 had increased odds ratios (ORs) for sore throat (OR 13.054; 95% confidence interval [CI] 2.750-61.977; p=0.001), nausea (OR 8.875; 95% CI 1.660-47.446; p=0.011), and lymphopenia (OR 3.575; 95% CI 1.355-9.430; p=0.010), but also had less hospitalizations (OR 0.355; 95% CI 0.138-0.916; p=0.032). The additional logistic regression analysis on patients with preexisting chronic conditions (n=108) showed that death as an outcome was significantly associated with pediatric severe acute respiratory syndrome (SARS) (OR 22.300; 95% CI 2.341-212.421; p=0.007) and multisystem inflammatory syndrome in children (MIS-C) (OR 11.261; 95% CI 1.189-106. 581; p=0.035).
CONCLUSIONS:Half of the laboratory-confirmed COVID-19 cases occurred in adolescents. Individuals belonging to this age group had an acute systemic involvement of SARS-CoV-2 infection. Pediatric SARS and MIS-C were the most important factors associated with the mortality rate in pediatric chronic conditions with COVID-19.
A pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared by the World Health Organization in March 2020. Children and adolescents with this infection usually present with milder infections and a better prognosis than adults (1–3).
Coronavirus disease 2019 (COVID-19) in pediatric populations accounts for up to 14% of all laboratory-confirmed cases (4). The spectrum of COVID-19 in children and adolescents ranges from asymptomatic to acute critical conditions. Death has rarely been reported and is mainly associated with pediatric severe acute respiratory syndrome (SARS) and multisystem inflammatory syndrome in children (MIS-C) (1–3,5–9).
Preexisting chronic conditions and comorbidities appear to be a risk factor for severe SARS-CoV-2 infection, with high death rates among children and adolescents (5,10). A case series involving pediatric populations of inpatients and outpatients in tertiary/quaternary hospitals for COVID-19 was reported in New York (11–13), Mexico (14), Brazil (2,3,7), Italy (15), Poland (16), the United Kingdom (17), Indonesia (18), and China (19), with a focus on risk factors for severity (2,11,12) and immunosuppression (7,14).
In addition, transmission of SARS-CoV-2 infection occurs more easily in secondary/high school adolescents than in primary school children (20). A recent study demonstrated increased hospitalization rates among adolescents, and approximately one-third of cases were admitted to pediatric intensive care units (PICUs), and 5% required invasive mechanical ventilation (21). However, to the best of our knowledge, few studies have evaluated the differences between children and adolescents with laboratory-confirmed SARS-CoV-2 infection, particularly in inpatients and outpatients followed up in a tertiary referral hospital.
Therefore, the objective of this study was to compare demographic, anthropometric, and clinical data, pediatric preexisting chronic conditions and comorbidities, laboratory tests, imaging abnormalities, treatments, and outcomes among children and adolescents with laboratory-confirmed COVID-19.
METHODSEthics Committee name and study protocol number: HCFMUSP - CAEE 4.889.659.
This was a cross-sectional study that included pediatric patients diagnosed with laboratory-confirmed COVID-19 between April 11, 2020, and April 22, 2021. The study site was the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), Brazil. This university referral hospital is the largest public and teaching hospital complex in Latin America (22–24) and worked as the main reference for pediatric patients with COVID-19 during the pandemic. It attended to patients from four institutes: Instituto Central, Instituto da Criança e do Adolescente, Instituto de Tratamento do Câncer Infantil, and Instituto do Coração, HCFMUSP.
The inclusion criteria were: 1) symptomatic inpatients and outpatients, 2) presence of clinical criteria for the screening of SARS-CoV-2 infection, and 3) age ≤18 years. The exclusion criteria included pregnant adolescents and subjects with incomplete medical record data. Children were defined as those aged <10 years, and adolescents were defined as those aged between ≥10 and <18 years.
The clinical criteria for screening of SARS-CoV-2 infection in children and adolescents were the presence of at least one of the following signs and symptoms during the COVID-19 pandemic: a) influenza-like disease in high-risk children (aged <5 years and/or with underlying conditions); b) pediatric SARS; c) fever without a source; d) fever and rash; e) fever in immunocompromised patients and without any other cause; f) MIS-C; and g) gastrointestinal or neurological signs or symptoms.
Between April 11, 2020 and April 22, 2021, 5,951 pediatric and adult patients with laboratory-confirmed COVID-19were hospitalized. Of these, 102/5,951 (1.7%) of the admissions occurred in neonates, children, and adolescents.
During this period, 3,962 SARS-CoV-2 detection samples were also processed in patients aged <18 years, and laboratory-confirmed COVID-19 occurred in 155 (4%) inpatients and outpatients. Six of the 155 pediatric patients with laboratory-confirmed COVID-19 were excluded because of incomplete medical records (n=3), pregnancy (n=2), and asymptomatic results confirmed by the pre-surgical evaluation (n=1).
Therefore, the final study group comprised 149 children and adolescents (n=97 inpatients and n=52 outpatients) with positive SARS-CoV-2 results: 122/149 (82%) were confirmed using real-time reverse transcription polymerase chain reaction (RT-PCR) assay on nasopharynx and oropharynx secretions, 23/149 (15%) had positive serological tests for SARS-CoV-2, and 4/149 (3%) children and adolescents were positive in both tests. Of these, 119/149 (80%) pediatric patients were diagnosed with COVID-19 in 2020, and 30/149 (20%) were diagnosed between January and April 22, 2021.
This study was approved by the Ethical Committee of our university hospital.
Data collection and study definitionsData were systematically retrieved from the medical records of each pediatric patient. The list of children and adolescents was revised by the Research Electronic Data Capture database of information for all pediatric cases with suspected COVID-19 at HCFMUSP, as well as the number of pediatric and adult outpatients with COVID-19 during the study period.
1) Demographics, anthropometric and clinical dataThe demographic data of patients with laboratory-confirmed COVID-19 included their current age, sex, and race. Body mass index values were calculated according using the patient's body weight divided by the square of their height and expressed in units of kg/m2. The following clinical manifestations in the pediatric diagnosis of COVID-19 were assessed: duration of signs or symptoms before diagnosis, fever, fever without a source, nasal discharge, sneezing, cough, sore throat, anosmia, dysgeusia, headache, myalgia, arthralgia, conjunctivitis, dyspnea, hypoxemia, nausea, vomiting, diarrhea, cutaneous rash, neurological abnormalities, and pneumonia. Pediatric SARS was defined according to the presence of a flu-like syndrome and at least one of the following: dyspnea, oxygen saturation below 95% in room air, or signs of respiratory distress (2). MIS-C diagnosis was established according to the Centers for Disease Control criteria (25). The severity of pediatric COVID-19 was classified as mild, moderate, severe, or critical according to Dong et al. (26).
2) Preexisting pediatric chronic conditions and comorbiditiesPreexisting pediatric chronic conditions in patients with laboratory-confirmed COVID-19 were defined according to whether the signs or symptoms lasted for more than three months, the diagnosis established by the physicians' scientific knowledge, and according to valid methods or instruments based on specific pediatric diagnostic criteria (27–29).
3) Laboratory tests, imaging abnormalities, treatments and outcomesLaboratory tests at the time of COVID-19 diagnosis included assessments of: hemoglobin concentration; leukocyte, neutrophil, lymphocyte, and thrombocyte counts; inflammatory biomarkers (C-reactive protein, erythrocyte sedimentation rate, fibrinogen, D- dimer, and ferritin); lactate dehydrogenase; serum albumin; aspartate and alanine aminotransferase; gamma-glutamyl transferase; alkaline phosphatase; blood urea; serum creatinine; triglycerides; muscle and brain creatine kinase and creatine kinase-isoenzymes; troponin T; prothrombin times; international normalized ratios; activated partial thromboplastin times; and hematuria, proteinuria, and pyuria. The examinations were conducted at the Clinical Laboratory of the Instituto Central, HCFMUSP.
Chest radiography and computed tomography were performed by a pediatric radiologist at the Instituto da Criança e do Adolescente do HCFMUSP, to search for abnormalities suggestive of SARS-CoV-2-related lesions, such as ground-glass opacities, consolidation, and linear and reticular opacities. Echocardiographic abnormalities were detected by two pediatric specialists at the Instituto da Criança e do Adolescente do HCFMUSP; abnormalities suggestive of SARS-CoV-2-related lesions, such as myocardial dysfunction, myocarditis, pericarditis, and/or coronary artery aneurysm z-scores ≥2.5, were identified.
Data on factors, such as transfusion of blood components (red blood, platelets, and plasma), oxygen therapy, concomitant antibiotics, oseltamivir, intravenous immunoglobulin, enoxaparin, aspirin, glucocorticoid use, intravenous methylprednisolone pulse therapy, dialysis, current use of immunosuppressants, and chemotherapy for cancer, were systematically recorded.
The following outcomes were also systematically evaluated in children and adolescents who were admitted to the hospital with COVID-19: hospitalization, duration of ward hospitalization, duration of PICU hospitalization, mechanical ventilation, duration of mechanical ventilation, use of vasoactive agents, arterial hypotension, shock, disseminated intravascular coagulation, thrombosis, and death.
4) Molecular and serological tests for SARS-CoV-2 detectionSARS-CoV-2 infection was confirmed by real-time RT-PCR or antibody testing. Real-time RT-PCR to assess SARS-CoV-2 genes was performed on respiratory samples at the molecular biology laboratory of our hospital (30). Tests for antibodies against the S proteins from the coronavirus spike were conducted in the Laboratório de Imunologia do Instituto Central do HCFMUSP using the following two assays: an enzyme-linked immunosorbent assay for detecting anti-SARS-CoV-2 immunoglobulin G (IgG) antibodies and a rapid immunochromatographic assay for detecting anti-SARS-CoV-2 IgM and IgG antibodies (31,32).
Statistical analysesBivariate analyses were performed to compare preexisting pediatric chronic conditions among children and adolescents with laboratory-confirmed COVID-19. A non-parametric test (Mann-Whitney U test) or a parametric test (Student's t-test) was used for continuous variables, and the results are presented as medians (minimum and maximum values) or means±standard deviations, respectively. Fisher's exact test or the Chi-square test was used for categorical variables. Logistic regression analyses were performed with age (<10 years and ≥10 years) or death as the dependent variable and independent variables that presented a statistical significance of p<0.2 in the bivariate analyses. The level of significance was established at 5%.
RESULTSTable 1 includes the demographic, anthropometric, and clinical data, as well as the preexisting pediatric chronic conditions and comorbidities present in children vs. those in adolescents with laboratory-confirmed COVID-19. The median duration of signs and symptoms before diagnosis was significantly lower in children than in adolescents [2 (0-60) vs. 3 (0-90) days, p=0.027]. The frequencies of sore throat, anosmia, dysgeusia, headache, myalgia, nausea, pediatric preexisting chronic conditions, autoimmune diseases, and the use of immunosuppressants were significantly reduced in children compared with adolescents (p<0.05, Table 1). No differences in the severity of pediatric COVID-19 were observed between the groups (p=0.287, Table 1).
Demographic, anthropometric and clinical data and pediatric preexisting chronic conditions in children (aged <10 years) vs. those in adolescents (aged ≥10 years) with laboratory-confirmed coronavirus disease 2019 (COVID-19).
| Variables | Children (n=74) | Adolescents (n=75) | p |
|---|---|---|---|
| Demographic and anthropometric data | |||
| Current age, years | 2 (0-9.83) | 14.75 (10.1-17.9) | <0.001 |
| Male sex | 42 (57) | 37 (49) | 0.364 |
| Caucasian race | 50 (67) | 53 (71) | 0.715 |
| Body mass index, kg/m2 (n=125) | 0.18±2.32 | 0.12±1.76 | 0.879 |
| Clinical data | |||
| Duration of signs/symptoms before diagnosis, days (n=148) | 2 (0-60) | 3 (0-90) | 0.027 |
| Fever | 56 (76) | 53 (71) | 0.490 |
| Fever without a source | 10 (13) | 5 (7) | 0.165 |
| Duration of fever, days (n=100) | 1 (1-8) | 2 (1-15) | 0.050 |
| Nasal discharge | 30 (40) | 29 (39) | 0.815 |
| Sneezing | 14 (19) | 11 (15) | 0.487 |
| Cough | 31 (42) | 39 (52) | 0.216 |
| Sore throat | 2 (3) | 22 (29) | <0.001 |
| Anosmia | 0 (0) | 12 (16) | <0.001 |
| Dysgeusia | 0 (0) | 6 (8) | 0.013 |
| Headache | 6 (8) | 29 (39) | <0.001 |
| Myalgia | 4 (5) | 25 (33) | <0.001 |
| Arthralgia | 0 (0) | 3 (4) | 0.082 |
| Conjunctivitis | 4 (5) | 5 (7) | 0.747 |
| Dyspnea | 26 (35) | 31 (41) | 0.436 |
| Hypoxemia | 23 (31) | 24 (32) | 0.904 |
| Nausea | 2 (3) | 15 (20) | 0.001 |
| Vomiting | 15 (20) | 11 (15) | 0.368 |
| Diarrhea | 8 (11) | 13 (17) | 0.253 |
| Cutaneous rash | 5 (7) | 6 (8) | 0.772 |
| Neurological abnormalities | 9 (12) | 6 (8) | 0.399 |
| Pneumonia | 16 (22) | 23 (31) | 0.209 |
| Severe acute respiratory syndrome | 12 (16) | 17 (23) | 0.302 |
| Multisystem inflammatory syndrome in children (MIS-C) | 3 (4) | 6 (8) | 0.312 |
| Pediatric preexisting chronic conditions | 53 (72) | 65 (87) | 0.024 |
| Diabetes mellitus | 0 (3) | 3 (4) | 0.082 |
| Arterial hypertension | 6 (8) | 9 (12) | 0.430 |
| Immunosuppressive conditions | 26 (35) | 41 (55) | 0.017 |
| Inborn errors of immunity | 2 (3) | 1 (1) | 0.552 |
| Solid organ transplantation | 1 (1) | 5 (7) | 0.099 |
| Hematopoietic stem cell transplantation | 2 (3) | 3 (4) | 0.660 |
| Cancer | 14 (19) | 14 (19) | 0.969 |
| Chronic kidney disease | 5 (7) | 6 (8) | 0.772 |
| Autoimmune conditions | 3 (4) | 10 (13) | 0.045 |
| Severity of pediatric COVID-19 | |||
| Mild | 37 (50) | 39 (52) | 0.287 |
| Moderate | 14 (19) | 9 (12) | |
| Severe | 16 (22) | 13 (17) | |
| Critical | 7 (9) | 14 (19) |
Results are presented as n (%), medians (minimum-maximum values), and means±standard deviations.
Table 2 illustrates the laboratory tests and imaging abnormalities in children and adolescents with laboratory-confirmed COVID-19. The median lymphocyte [2,610 (0-17,860) vs. 1,315 (0-9,890)/mm3, p<0.001] and thrombocyte counts (254,439±136,531 vs. 197,054±115,116/mm3, p=0.003) were significantly higher in children than in adolescents, whereas fibrinogen [284 (0-766) vs. 388.5 (0-1,274) mg/dL, p=0.023] and ferritin levels [118 (0-15,179) vs. 358 (37-35,976) ng/mL, p=0.016] were significantly reduced in the first group (Table 2).
Laboratory tests and imaging abnormalities in children (aged <10 years) vs. those in adolescents (≥10 years) with laboratory-confirmed coronavirus disease 2019 (COVID-19).
| Variables | Children (n=74) | Adolescents (n=75) | p |
|---|---|---|---|
| Hematological parameters | |||
| Hemoglobin concentration, g/dL (n=126) | 11.28±2.09 | 11.55±2.19 | 0.471 |
| Hemoglobin <10 g/dL (n=126) | 17/66 (26) | 11/60 (18) | 0.317 |
| Leucocyte count/mm3 (n=126) | 8,210±5,815 | 7,377±4,583 | 0.377 |
| Leucopenia <4,000/mm3 (n=126) | 7/66 (11) | 14/60 (23) | 0.056 |
| Neutrophil count/mm3 (n=125) | 3,200 (0-28,768) | 3,905 (0-27,900) | 0.064 |
| Neutropenia <1,000/mm3 (n=125) | 8/65 (12) | 5/60 (8) | 0.467 |
| Lymphocyte count/mm3 (n=125) | 2,610 (0-17,860) | 1,315 (0-9,890) | <0.001 |
| Lymphopenia <1,500/mm3 (n=125) | 15/65 (23) | 33/60 (55) | <0.001 |
| Thrombocyte count/mm3 (n=125) | 254,439±136,531 | 197,054±115,116 | 0.003 |
| Thrombocytopenia <100,000/mm3 (n=125) | 8/66 (12) | 13/59 (22) | 0.139 |
| Inflammatory markers | |||
| C-reactive protein, mg/L (n=118) | 10.96 (0.3-311) | 20.8 (0.3-580.4) | 0.050 |
| C-reactive protein >30 mg/L (n=118) | 21/61 (34) | 25/57 (44) | 0.294 |
| ESR, mm/first hour (n=23) | 36.6 (0-140) | 35 (0-140) | 0.804 |
| Fibrinogen, mg/dL (n=65) | 284 (0-766) | 388.5 (0-1,274) | 0.023 |
| D-dimer, ng/mL (n=91) | 1,286 (190-95,040) | 1,043 (190-86,900) | 0.584 |
| D-dimer >1000 ng/mL (n=91) | 26/45 (58) | 24/46 (52) | 0.591 |
| Ferritin, ng/mL (n=96) | 118 (0-15,179) | 358 (37-35,976) | 0.016 |
| Other exams | |||
| Lactate dehydrogenase, U/L (n=76) | 316.5 (4-3,040) | 335.5 (0-6,000) | 0.621 |
| Serum albumin, g/dL (n=71) | 3.70±0.73 | 3.42±0.73 | 0.121 |
| Aspartate aminotransferase, U/L (n=111) | 32.5 (13-377) | 26 (7-2,002) | <0.001 |
| Alanine aminotransferase, U/L (n=111) | 22 (6-495) | 24 (5-3,338) | 0.885 |
| Gamma-glutamyl transferase, U/L (n=61) | 33 (6-1,262) | 92.5 (15-483) | 0.116 |
| Alkaline phosphatase, U/L (n=58) | 163 (77-834) | 133 (69-545) | 0.038 |
| Blood urea, mg/dL (n=114) | 18 (6-106) | 23 (4-186) | 0.012 |
| Serum creatinine, mg/dL (n=114) | 0.34 (0.03-17) | 0.56 (0.17-10.06) | <0.001 |
| Triglycerides, mg/dL (n=31) | 93 (41-750) | 146 (59-308) | 0.477 |
| CK, U/L (n=62) | 101 (31-1,008) | 90 (13-3,331) | 0.703 |
| CK-MB, ng/mL (n=52) | 2.85 (0.64-14.7) | 1.34 (0.3-28.94) | 0.010 |
| Troponin T, ng/mL (n=86) | 0.011 (0.003-0.08) | 0.009 (0.003-1.05) | 0.941 |
| Prothrombin time, s (n=92) | 13.1 (1.28-16.1) | 13.35 (10.8-38.2) | 0.061 |
| INR (n=92) | 1.01 (0.9-1.23) | 1.08 (0.9-3.13) | 0.013 |
| Activated partial thromboplastin time, s (n=91) | 32.17±7.16 | 36.28±7.32 | 0.008 |
| Hematuria >5 erythrocytes/mL (n=66) | 6/39 (15) | 7/27 (26) | 0.290 |
| Proteinuria >0.5 g/day (n=57) | 2/34 (6) | 4/23 (17) | 0.165 |
| Chest X-ray abnormalities (n=90) | 25/46 (54) | 22/44 (50) | 0.680 |
| Pulmonary CT abnormalities (n=41) | 15/20 (75) | 16/21 (76) | 0.929 |
| Cardiac alterations on echocardiogram (n=68) | 14/34 (41) | 13/34 (38) | 0.802 |
Results are presented as n (%), medians (minimum-maximum values), and means±standard deviations; ESR, erythrocyte sedimentation rate; CK, creatine kinase; CK-MB, muscle and brain creatine kinase-isoenzyme; INR, international normalized ratio; CT, computed tomography.
A comparison of the treatments and outcomes between children and adolescents with laboratory-confirmed COVID-19 are shown in Table 3. The frequencies of enoxaparin (8% vs. 20%, p=0.037), current immunosuppressants (19% vs. 39%, p=0.008), vasoactive agents (4% vs. 13%, p=0.045), arterial hypotension (0% vs. 16%, p<0.001), and shock (7% vs. 15%, p=0.024) were significantly lower in children than in adolescents, whereas hospitalization was significantly higher in children (74% vs. 56%, p=0.016).
Treatments and outcomes in children (aged <10 years) vs. those in adolescents (aged ≥10 years) with laboratory-confirmed coronavirus disease 2019 (COVID-19).
| Variables | Children (n=74) | Adolescents (n=75) | p |
|---|---|---|---|
| Treatments | |||
| Blood product transfusion | 9 (12) | 11 (15) | 0.654 |
| Red blood cell transfusion | 9 (12) | 10 (13) | 0.830 |
| Platelet transfusion | 4 (5) | 8 (11) | 0.238 |
| Plasma transfusion | 2 (3) | 2 (3) | 0.989 |
| Oxygen therapy | 23 (31) | 27 (36) | 0.525 |
| Antibiotics | 39 (53) | 40 (53) | 0.939 |
| Oseltamivir | 18 (24) | 23 (31) | 0.386 |
| Intravenous immunoglobulin | 4 (5) | 6 (8) | 0.527 |
| Enoxaparin | 6 (8) | 15 (20) | 0.037 |
| Aspirin | 1 (1) | 5 (7) | 0.099 |
| Glucocorticoids | 15 (20) | 17 (23) | 0.722 |
| Intravenous methylprednisolone pulse therapy | 4 (5) | 2 (3) | 0.395 |
| Dialysis for acute renal injury or shock | 2 (3) | 4 (5) | 0.414 |
| Current immunosuppressants | 14 (19) | 29 (39) | 0.008 |
| Current chemotherapy for cancer | 10 (13) | 10 (13) | 0.974 |
| Outcomes | |||
| Hospitalization | 55 (74) | 42 (56) | 0.019 |
| Duration of ward hospitalization, days (n=89) | 6.5 (1-120) | 8 (1-67) | 0.813 |
| Duration of PICU hospitalization, (n=30) | 5 (1-60) | 7 (2-46) | 0.620 |
| Mechanical ventilation | 7 (9) | 13 (17) | 0.876 |
| Duration of mechanical ventilation, (n=32) | 5 (3-15) | 6.5 (2-15) | 0.915 |
| Use of vasoactive agents | 3 (4) | 10 (13) | 0.045 |
| Arterial hypotension | 0 (0) | 12 (16) | <0.001 |
| Shock | 5 (7) | 11 (15) | 0.024 |
| Disseminated intravascular coagulation | 0 (0) | 5 (7) | 0.876 |
| Thrombosis | 1 (1) | 5 (7) | 0.099 |
| Death | 2 (3) | 7 (9) | 0.089 |
Results are presented in n (%), medians (minimum-maximum values), and means±standard deviations; PICU, pediatric intensive care unit.
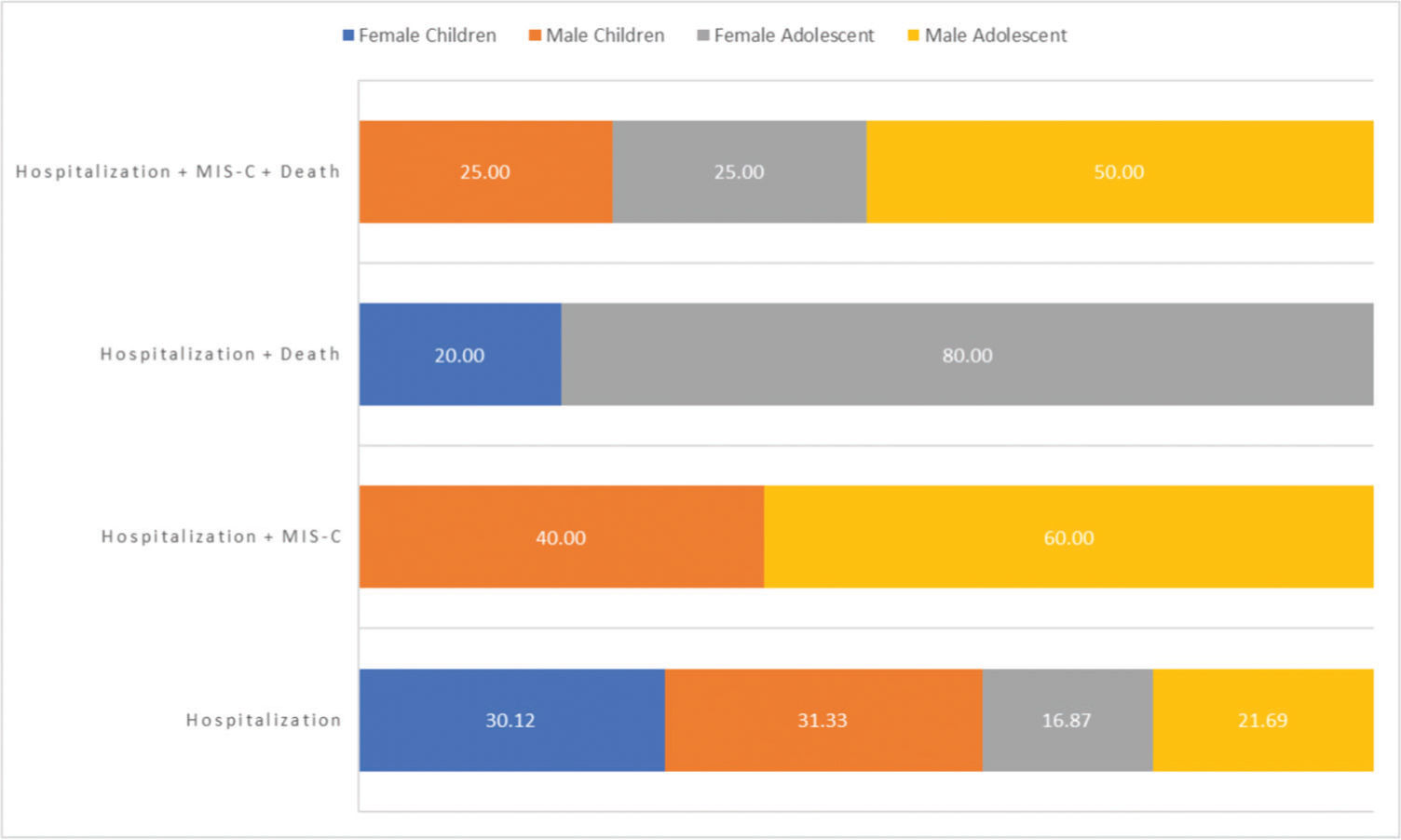
Figure 1 summarizes the demographic data and outcomes of children vs. those in adolescents with laboratory-confirmed COVID-19. The frequency of hospitalization and death was significantly lower in female children than in female adolescents (20% vs. 80%, p=0.019). The frequency of hospitalization was significantly higher in female and male children than in female and male adolescents (30% vs. 31% vs. 17% vs. 22%, p=0.004). No differences in other demographic data or in the combination of outcomes were observed (p>0.05, Figure 1).
Demographic data and outcomes in children (aged <10 years) vs. those in adolescents (aged ≥10 years) with laboratory-confirmed coronavirus disease 2019 (COVID-19). MIS-C, multisystem inflammatory syndrome in children; hospitalization+MIS-C+death (p=0.787), hospitalization+death (p=0.019), hospitalization+MIS-C (p=0.133), and hospitalization (p=0.004).
Logistic regression analysis showed that adolescents with laboratory-confirmed COVID-19 had increased odds ratios (ORs) of sore throat (OR 13.054; 95% confidence interval [CI] 2.750-61.977; p=0.001), nausea (OR 8.875; 95% CI 1.660-47.446; p=0.011), and lymphopenia (OR 3.575; 95% CI 1.355-9.430; p=0.010), although they also had fewer hospital admissions (OR 0.355; 95% CI 0.138-0.916; p=0.032).
A further logistic regression analysis, which included death as the dependent variable and age, pediatric SARS, MIS-C, and preexisting chronic conditions as the independent variables, showed that the presence of death was significantly associated with pediatric SARS (OR 34.269; 95% CI 3.754-312.802; p=0.002) and MIS-C (OR 11.555; 95% CI 1.478-90.327; p=0.020). An additional logistic regression analysis (including only patients with preexisting chronic conditions (n=108), which used death as the dependent variable and age, pediatric SARS, and MIS-C as the independent variables, showed that the presence of death was significantly associated with pediatric SARS (OR 22.300; 95% CI 2.341-212.421; p=0.007) and MIS-C (OR 11.261; 95% CI 1.189-106.581; p=0.035).
DISCUSSIONThis study showed that laboratory-confirmed COVID-19 occurred in 50% of the studied adolescents. Individuals belonging to this age group had more intense multisystemic involvement of SARS-CoV-2 infection than children, mainly presenting with sore throat, nausea, and lymphopenia. Pediatric SARS and MIS-C were the most important factors associated with mortality in patients with chronic diseases and COVID-19.
The main strength of this study was the inclusion of pediatric patients with laboratory-confirmed SARS-CoV-2 infection, involving almost 80% of pediatric chronic conditions. Another strength was the use of a standardized database to minimize bias. Our tertiary university hospital is the largest public funded in Latin America (2,3,22). During the COVID-19 pandemic in Sao Paulo, which is the most populous city in Brazil, this academic health complex, through its Crisis Management Committee, guided the hospitalization of pediatric and adult patients with moderate to critical COVID-19. This large hospital complex provided exclusive care for inpatients with COVID-19, with a total of 900 beds and more than 300 ICU beds for adults and children/adolescents in the peak months and during the pandemic waves (23,24,33). Another advantage of this study was the pediatric multidisciplinary team that was available to support inpatients and outpatients with COVID-19; it involved physicians and fellows of various pediatric subspecialties, as well as nurses, physiotherapists, psychologists, nutritionists, biologists, social workers, clinical pharmacists, and physical educators. This group was selected to attend and to conduct specific research involving neonates, children, and adolescent inpatients and outpatients with SARS-CoV-2 infection (2,4,6-9,34-38), as well as the implementation of an innovative telemedicine program that was established during the COVID-19 pandemic (39).
We confirmed that hospitalizations because of COVID-19 affected the pediatric population less frequently than adults, as has been previously reported (14). In fact, admission for pediatric COVID-19 occurred in 1.7% of the evaluated patients during one year of the pandemic in our university hospital. Interestingly, only 4% of them had laboratory-confirmed COVID-19 of a total of almost 4,000 samples from patients presenting with warning signs for the screening of SARS-CoV-2 infection, reinforcing the rarity of this infection during this year in our pediatric population.
We extend the previous reports of laboratory-confirmed pediatric COVID-19 cases, where adolescents had a more intense, acute symptomatic, and systemic involvement of COVID-19 than children but also a lower rate of hospitalization. The higher frequencies of sore throat and nausea, as well as anosmia, dysgeusia, headache, and myalgia, in adolescents with SARS-CoV-2 infection may be related to the high reporting rates of these signs and symptoms in the individuals belonging to this age group, as they are more aware of COVID-19 signs and symptoms than younger children. Furthermore, preexisting chronic conditions, particularly the use of immunosuppressants and presence of autoimmune diseases, appear to have contributed to the clinical spectrum of COVID-19 in adolescents (1,2,4,8,33,36).
Furthermore, lymphopenia is a distinct laboratory feature of adolescents with COVID-19. This finding may be related to the higher frequencies of immunosuppressive conditions observed in our teenagers at the time of this emerging infectious disease diagnosis, a possible relationship with the disease activity, or the use of immunosuppressants or chemotherapy drugs. A recent pediatric COVID-19 meta-analysis revealed that lymphopenia and leukopenia were the most common white cell abnormalities (40).
Shock and arterial hypotension are striking complications of the disease course in adolescents with SARS-CoV-2 infection. Individuals belonging to this age group also required more enoxaparin and vasoactive agents, indicating a critical COVID-19 condition among adolescents.
Importantly, our data showed a mortality rate of 6%, similar to that in other studies in tertiary hospitals that ranged from 0% to 5% (12,14,16,18). In contrast, a cross-sectional study conducted in the first semester of 2020 using Brazilian data from the National Epidemiological Surveillance Information System showed a higher death rate of 15% in 2,570 confirmed pediatric COVID-19 cases with SARS (5).
Pediatric SARS and MIS-C were the most important causes of death in both children and adolescents with COVID-19, as has also been reported in other studies (2,18). Reinforcing these results, the autopsy features of five of our patients with SARS-CoV-2 infection showed primary pulmonary disease, with SARS and diffuse alveolar damage present in some patients and MIS-C with multiple organ involvement in another patient (41). Interestingly, the isolation of SARS-CoV-2 with cellular ultrastructural changes in several organs of patients with MIS-C supports the idea of a direct effect of SARS-CoV-2 on tissues and relevant role of this agent in the pathogenesis of this severe COVID-19 involvement (41,42).
Our study has limitations, such as a population selection bias resulting from the study being conducted in a tertiary referral hospital for children, including patients with a more severe spectrum of COVID-19. In addition, there were no data on cytokine levels, which may have played a role in the outcomes of patients with COVID-19 and MIS-C. The SARS-CoV-2 lineage P.1 (gamma variant of concern), which was discovered in Manaus, Brazil in early January 2021, was responsible for the second wave across the country and was not evaluated in this study (43,44). However, only 20% of our cases were diagnosed in the first four months of 2021. In fact, during that period, COVID-19 vaccination was initiated in adults; thus, this may have contributed to the decrease in SARS-CoV-2 transmission in communities, families, and hospital environments.
In conclusion, laboratory-confirmed COVID-19 occurred in 50% of the adolescents in this study. Adolescents have a more intense, acute, and systemic involvement of SARS-CoV-2 infection. Pediatric SARS and MIS-C were the most important factors associated with the mortality rates in pediatric chronic conditions with COVID-19.
HC-FMUSP Pediatric COVID Study GroupAdriana Pasmanik Eisencraft, Alfio Rossi Junior, Alice Lima Fante, Aline Pivetta Cora, Amelia Gorete A. de Costa Reis, Ana Paula Scoleze Ferrer, Anarella Penha Meirelles de Andrade, Andreia Watanabe, Angelina Maria Freire Gonçalves, Aurora Rosaria Pagliara Waetge, Camila Altenfelder Silva, Carina Ceneviva, Carolina dos Santos Lazari, Deipara Monteiro Abellan, Emilly Henrique dos Santos, Ester Cerdeira Sabino, Fabíola Roberta Marim Bianchini, Flávio Ferraz de Paes Alcantara, Gabriel Frizzo Ramos, Gabriela Nunes Leal, Isadora Souza Rodriguez, João Renato Rebello Pinho, Jorge David Avaizoglou Carneiro, Jose Albino Paz, Juliana Carvalho Ferreira, Juliana Ferreira Ferranti, Juliana de Oliveira Achili Ferreira, Juliana Valéria de Souza Framil, Katia Regina da Silva, Kelly Aparecida Kanunfre, Karina Lucio de Medeiros Bastos, Karine Vusberg Galleti, Lilian Maria Cristofani, Lisa Suzuki, Lucia Maria Arruda Campos, Maria Beatriz de Moliterno Perondi, Maria de Fatima Rodrigues Diniz, Maria Fernanda Mota Fonseca, Mariana Nutti de Almeida Cordon, Mariana Pissolato, Marina Silva Peres, Marlene Pereira Garanito, Marta Imamura, Mayra de Barros Dorna, Michele Luglio, Mussya Cisotto Rocha, Nadia Emi Aikawa, Natalia Viu Degaspare, Neusa Keico Sakita, Nicole Lee Udsen, Paula Gobi Scudeller, Paula Vieira de Vincenzi Gaiolla, Rafael da Silva Giannasi Severini, Regina Maria Rodrigues, Ricardo Katsuya Toma, Ricardo Iunis Citrangulo de Paula, Patricia Palmeira, Silvana Forsait, Sylvia Costa Lima Farhat, Tânia Miyuki Shimoda Sakano, Vera Hermina Kalika Koch, Vilson Cobello Junior. All of the members are from Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, SP, BR.
AUTHOR CONTRIBUTIONSAll named authors approved the final draft of the manuscript, approved the submission to the journal, and are willing to take responsibility for it in its entirety. All authors contributed substantially to the conception and design of the study and in the analysis and interpretation of the data. All authors revised the manuscript critically and approved its final version.
We thank Amaro Nunes Duarte Neto, Anna Sara Levin, Esper Georges Kallas, Eurípedes Constantino Miguel, Geraldo Filho Busatto, Guilherme Vanoni Polanczyk, Sônia Regina Testa da Silva Ramos, Marisa Dolhnikoff, Paulo Hilario do Nascimento Saldiva and the members of the HCFMUSP COVID-19 Study Group: Aluisio C Segurado, Anna Miethke-Morais, Amanda C Montal, Carolina Carmo, Carolina dos Santos Lázari, Clarice Tanaka, Eloisa Bonfá, Edivaldo M. Utiyama, Fabiane Yumi Ogihara Kawano, Izabel Marcilio, Izabel Cristina Rios, Julio F. M. Marchini, Leila Harima, Maria Amélia de Jesus, Marcelo C. Rocha, Maria Cristina Peres Braido Francisco, Marcello M. C. Magri, Marjorie F. Silva, Maura Salaroli Oliveira, Solange R. G. Fusco, Tarcisio E. P. Barros-Filho, and Thaís Guimarães. Funding: This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 304984/2020-5 to CAS), Fundação de Amparo è Pesquisa do Estado de São Paulo (FAPESP 2015/03756-4 to CAS), and by Núcleo de Apoio è Pesquisa “Saúde da Criança e do Adolescente” da USP (NAP-CriAd) to CAS.
No potential conflict of interest was reported.