Most lung transplants are obtained from brain-dead donors. The physiopathology of brain death involves hemodynamics, the sympathetic nervous system, and inflammatory mechanisms. Administering methylprednisolone 60 min after inducing brain death in rats has been shown to modulate pulmonary inflammatory activity. Our objective was to evaluate the effects of methylprednisolone on transplanted rat lungs from donors treated 60 min after brain death.
METHODS:Twelve Wistar rats were anesthetized, and brain death was induced. They were randomly divided into two groups (n = 6), namely a control group, which was administered saline solution, and a methylprednisolone group, which received the drug 60 min after the induction of brain death. All of the animals were observed and ventilated for 2 h prior to being submitted to lung transplantation. We evaluated the hemodynamic and blood gas parameters, histological score, lung tissue levels of thiobarbituric acid-reactive substances, level of superoxide dismutase, level of tumor necrosis factor-alpha, and level of interleukin-1 beta.
RESULTS:After transplantation, a significant reduction in the levels of tumor necrosis factor-alpha and IL-1β was observed in the group that received methylprednisolone (p = 0.0084 and p = 0.0155, respectively). There were no significant differences in tumor necrosis factor-alpha and superoxide dismutase levels between the control and methylprednisolone groups (p = 0.2644 and p = 0.7461, respectively). There were no significant differences in the blood gas parameters, hemodynamics, and histological alterations between the groups.
CONCLUSION:The administration of methylprednisolone after brain death in donor rats reduces inflammatory activity in transplanted lungs but has no influence on parameters related to oxidative stress.
The availability of donor organs remains a challenge for transplantation programs worldwide. This issue is a particular problem for lung transplantation, which is the ultimate option for patients with end-stage pulmonary disease. Approximately 5%-20% of donors who elect for organ retrieval have lungs that are considered viable for lung transplantation. However, the number of patients enrolled on waiting lists has been increasing 1-3.
Brain-dead donors still represent the main source of organs for transplantation. It is well known that brain death compromises the viability of transplanted solid organs, and it can affect lung donor viability through sympathetic, hemodynamic, and inflammatory mechanisms that lead to adrenergic storm and neurogenic pulmonary edema 4. In addition, these lungs are at risk for acute lung injury secondary to trauma, prolonged mechanical ventilation, transfusion, ischemia, aspiration, and infection 4,5.
Currently, there is mounting evidence to suggest that organ grafts are not immunologically inert. Donor risk factors, such as previous disease, age, cause of death, donor management, and, most importantly, brain death, reprogram the graft into an immunologically active organ 5. Accordingly, treating potential brain-dead donors may reduce immune activation and improve the condition of the lung that is to be transplanted 6.
The administration of systemic corticosteroids to brain-dead donors is known to be beneficial, mostly because it modulates the systemic inflammatory response caused by brain death 7. This modulation improves graft viability by reducing both the release of pro-inflammatory molecules and the production of leukocyte adhesion molecules, thereby increasing the clearance of alveolar fluids 7,8. However, the optimal timing of the administration of corticosteroids during brain death remains unknown. Previously, we showed that in a model of brain death, the administration of methylprednisolone at 5 and 60 min reduced the levels of TNF-α to a similar extent 9. In the present study, we hypothesized that the transplanted lungs from donors treated with methylprednisolone after 60 min of brain death would exhibit less inflammatory activity than those of untreated donors.
MATERIALS AND METHODSThe Ethical and Research Committee of Hospital de Clínicas de Porto Alegre approved the protocols used in this study. All of the animals received humane care that was in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, published by the National Institutes of Health, Publication No. 86-23, revised 1996).
Surgical procedureTwelve male rats, weighing 300-400 g each, underwent general anesthesia induced by an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (15 mg/kg). Anesthesia was followed by a tracheostomy using an indwelling 14-gauge cannula (Abbocath® #14; Abbott Laboratories, Abbott Park, IL, EUA) and ventilation at a rate of 70-80 breaths/min with a tidal volume of 10 ml/kg of inspired room air (Harvard Rodent Ventilator, model 683; Harvard Apparatus Co., Millis, MA). The right carotid artery was dissected and cannulated using a 24-gauge cannula (Becton Dickinson, Franklin Lakes, NJ, USA) to record the mean arterial pressure (MAP) and heart rate (HR) (Sirecust 730 Siemens, Solna, Sweden). The right jugular vein was also dissected and cannulated in the same manner. Normal saline was used to flush the lines, with a total volume of 5 ml/kg/h used in all of the animals, and a warmed surgical table was used to maintain the body temperature at 37°C during the procedure.
Two hours following brain death, the donor rat lungs were flushed in an antegrade manner with 20 ml of cold (4°C), low-potassium dextran solution (LPD-Perfadex®; Vitrolife, Göteborg, Sweden) at a pressure of 20 cmH2O via the pulmonary artery, as described elsewhere 10. The heart-lung block was extracted with the lungs inflated at the end-tidal volume. The left lung graft was isolated, prepared, and stored in LPD at 4°C for 90 min.
Twelve recipients were anaesthetized, intubated (14-gauge catheter), and ventilated. Then, they underwent a left thoracotomy, and the pulmonary vessels and left bronchus were anastomosed using a standard cuff technique 10. The arterial clamps were released approximately 3 h after harvest. The recipient animals were then observed for 2 h. At the end of the observation period, the pulmonary hilum was clamped, and the lower half of the lung was immediately snap-frozen in liquid nitrogen and stored at −80°C. The upper half of the lung was embedded in 10% formaldehyde for histopathology.
Induction of brain deathThe brain death model used here has previously been described in detail 11,12. Briefly, a frontolateral trepanation (1×1 mm with a dental drill) was performed, and a 14-gauge Fogarty balloon catheter (Baxter Health Care Corp., Irvine, CA, USA) was introduced into the extradural space with the tip pointed caudally. The balloon was inflated with 0.75 ml of water for 1 min, producing a sudden increase in intracranial pressure, which resulted in rapid, progressive brain injury leading to immediate brain death. A sharp rise, followed by a subsequent drop, of blood pressure and heart rate defined the initiation of brain death. The presence of brain death was confirmed by the absence of corneal reflexes and by the apnea test.
Study groupsAfter the initial procedures, the animals were randomly assigned to two brain-dead donor groups, namely the control (n = 6) and corticosteroid (n = 6) groups. In the control group (CONTROL), an IV bolus of 0.9% saline solution (0.3 ml) was administered 60 min after the induction of brain death. In the corticosteroid group (MET), an IV bolus of methylprednisolone (30 mg/kg) diluted in 0.2 ml of normal saline was administered 60 min after the induction of brain death. The timing and dose of methylprednisolone were based on a previous study 9.
SamplingAll of the donor and recipient animals were monitored for 120 min and were submitted to the same ventilation procedures. Arterial blood samples were obtained at the time of insertion of the arterial line (basal) and at 60 and 120 min in the donor groups. In the recipient groups, the samples were drawn at 5 and 120 min after transplantation for blood gas analysis.
The lower half of the left lung was snap-frozen in liquid nitrogen and stored at −80°C for analyses of lipid peroxidation and superoxide dismutase (SOD) activity and for the protein quantification of TNF-α and IL-1β 13.
Pulmonary TNF-α and IL-1β assayAfter the samples were thawed, a 96-well plate was coated with monoclonal antibodies against TNF-α and IL-1β. The wells were filled with either 100 µl of homogenized lung (diluted 1∶2), 100 µl of positive or negative controls, or 100 µl of recombinant TNF-α or IL-1β at concentrations established by the manufacturer (Creative Biomart, NY, USA). Then, 100 µl of polyclonal anti-TNF-α and anti-IL-1β conjugated to peroxidase was added to the wells, and the samples were incubated for 3 h at room temperature. Following the incubation, the plate was washed four times with a detergent solution. The color change was then induced by adding hydrogen peroxide (0.02%) and tetramethylbenzene (2%). The reaction was interrupted 30 min later using sulfuric acid (1 M). The color intensity was assessed by obtaining optical density measurements using an ELISA automatic reader (Titertek Multiskan®) at a wavelength of 450 nm. The TNF-α and IL-1β concentrations in the homogenized lung samples were calculated based on the results of a standard curve.
Determination of thiobarbituric acid reactive substances (TBARS)The products generated by lipid peroxidation were quantified by the TBARS reaction using 3 mg of protein per sample. The samples were first incubated at 90°C for 30 min. Then, 500 µl of 0.37% thiobarbituric acid and 15% trichloroacetic acid was added to the samples, and they were centrifuged at 4°C at 2000xg for 15 min. The absorbance was then determined by spectrophotometry at 535 nm 14.
Superoxide dismutase (SOD)The activity of SOD was determined using the pulse radiolytic method based on the auto-oxidation of epinephrine, as described by Misra and Fridovich 15.
HistologyThe portion of lung fixed in formalin was embedded in paraffin, cut into 3-mm sections, and stained with hematoxylin and eosin. A pathologist blinded to the experimental protocol performed a quantitative examination under light microscopy. Each lung sample was examined under both low- and high-power fields, and 20 fields were randomly selected and analyzed. The severity of the histological lesions was assigned a score based on five parameters, namely intra-alveolar edema, hyaline membrane formation, hemorrhage, focal alveolar collapse or consolidation, and epithelial desquamation or the necrosis of airways or alveoli. Each parameter was evaluated semi-quantitatively according to the following scale: 0 = absent, 1 = mild, 2 = moderate, and 3 = prominent. For each animal, the scored value of each parameter was added to produce a final score 16.
Statistical analysisAll of the data collected from the experiments were coded, recorded, and analyzed using SPSS 16.0 for Windows (Chicago, IL, USA). To analyze the differences in the demographic variables over time in each group, the Wilcoxon nonparametric test was used. To analyze the differences in the demographic variables between groups, the Mann-Whitney U nonparametric test was used. For each test, the data are expressed as the median ± the interquartile range (IR), and p<0.05 was accepted as statistically significant. For the cytokine, TBARS, SOD, and histology analyses, the Student's t-test was used; the data are expressed as the mean value ± standard error (SE), and p<0.05 was accepted as statistically significant.
RESULTSThere were no differences between the two groups with respect to procedure time, blood gas measurements, and mean arterial pressure. In the donor rats, the mean arterial pressure decreased significantly in both groups after the induction of brain death (p<0.05) (Table1). There were no differences between the two groups with respect to MAP and blood gas analyses following transplantation (Table2).
The level of arterial blood gases and the mean arterial pressure in donor rats before and 2 h following brain death.
| Donors | ||||||
|---|---|---|---|---|---|---|
| Control Group | Methylprednisolone Group | |||||
| Baseline (median±IR) | 2 h after BD (median±IR) | p-value | Baseline (median±IR) | 2 h after BD (median±IR) | p-value | |
| PO2 | 84.53±7.07 | 73.40±26.42 | 0.48 | 100.28±32.51 | 67.30±17.95 | 0.055 |
| PCO2 | 42.53±14.68 | 44.38±22.44 | 0.87 | 32.00±6.27 | 37.10±12.39 | 0.38 |
| MAP | 99.96±28.47 | 54.07±16.13 | 0.006* | 89.68±16.41 | 59.62±10.73 | 0.003* |
PaO2 = partial pressure of arterial oxygen; PaCO2 = partial pressure of arterial carbon dioxide; MAP = mean arterial pressure. Values are expressed as the median ± interquartile of the median. * = statistical significance, as defined by p<0.05 according to the Mann-Whitney U test.
The levels of arterial blood gases and the mean arterial pressure in recipients at baseline and 2 h following lung transplantation.
| Recipients | ||||
|---|---|---|---|---|
| Control Group | Methylprednisolone Group | p-value | ||
| PO2 | Baseline (median±IR) | 226.27±132.99 | 191.30±88.49 | 0.631 |
| After 2 h (median±IR) | 242.55±138.15 | 288.98±125.35 | 0.465 | |
| PCO2 | Baseline (median±IR) | 44.87±6.76 | 38.85±9.88 | 0.297 |
| After 2 h (median±IR) | 40.52±19.76 | 56.74±41.62 | 0.715 | |
| MAP | Baseline (median±IR) | 88.67±18.47 | 69.89±16.75 | 0.077 |
| After 2 h (median±IR) | 55.44±19.74 | 60.72±14.22 | 0.522 |
PaO2 = partial pressure of arterial oxygen; PaCO2 = partial pressure of arterial carbon dioxide; MAP = mean arterial pressure; # = Mann-Whitney U test. Values are expressed as the median ± interquartile range of the median.
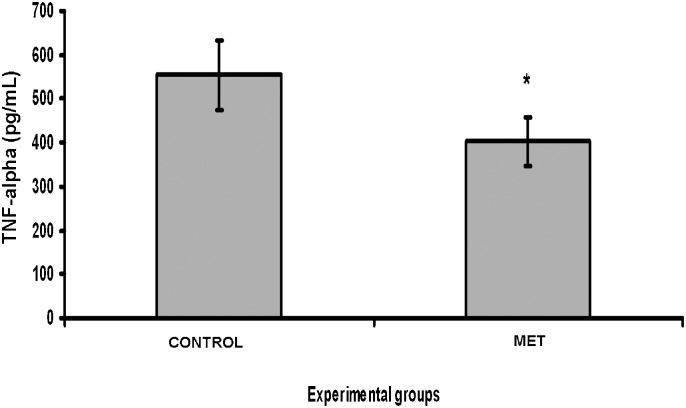
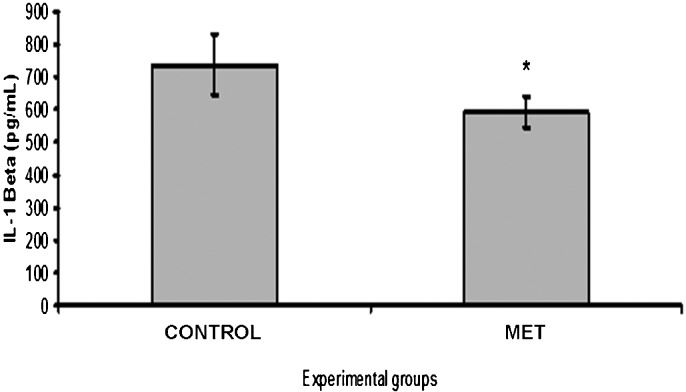
There were no significant differences in the results of the TBARS and SOD assays between the MET and CONTROL groups (p = 0.2644 and p = 0.7461, respectively). The TNF-α and IL-1β tecidual concentrations were significantly lower in the MET group (p = 0.0084 and p = 0.0155, respectively) (Figures1 and 2).
Pulmonary tumor necrosis factor-alpha assay. After transplantation, there was a significant decrease in the levels of tumor necrosis factor-alpha in the methylprednisolone group compared with the control group (*p = 0.0084). The values are expressed as the mean ± standard error of the mean.
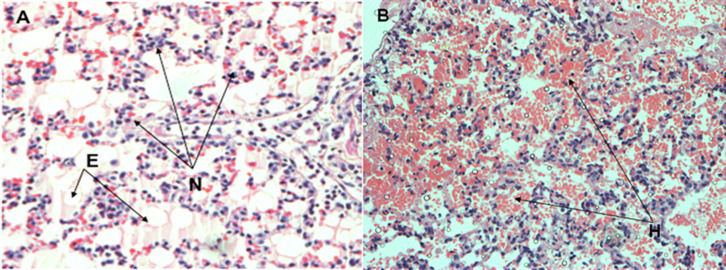
The severity of the histological damage, as measured by the histology score (HIS), was similar between the CONTROL and MET groups. The predominant finding was mild focal alveolar collapse and congestion (Figure3).
The main histological findings in the control and methylprednisolone groups after lung transplantation. The lungs from the methylprednisolone group (A) showed the recruitment of septal neutrophils (N) and edema (E). The lungs from the control group (B) showed areas of hemorrhage (H) (200× magnification).
While the early survival rates of patients after lung transplantation have improved (1), their long-term survival remains challenging 2,3. Immunological events are considered to be key factors in this scenario, and these events may be aggravated by the alterations triggered by brain death 17. The intensive care of potential donors may reflect the outcome after transplantation, and the administration of methylprednisolone has been suggested to be beneficial in promoting organ maintenance in brain-dead donors 18.
Currently, the use of corticosteroids in potential donors remains controversial, and there is no consensus regarding the time of the first administration or the ideal dose required to achieve an adequate anti-inflammatory effect 19. While several studies have shown that resuscitative hormonal protocols are promising, to date, no standard protocols for lung retrieval have been accepted 19-20. The use of corticosteroids in brain-dead donors is justified by two potential beneficial effects, namely a reduction in the inflammatory response and a replacement of the blood hormonal levels. Faropoulos et al. observed that in a baboon model, cortisol levels were reduced 15-45 min after the induction of brain death and almost disappeared after approximately 4 h 21.
In our previous study, we found that both the early (5 min) and late (60 min) administration of methylprednisolone reduced TNF-α to levels similar to those present 2 h after brain death. In the present study, we administered methylprednisolone after 60 min of brain death to simulate the clinical scenario, in which the prompt diagnosis of brain death is usually delayed. We retrieved donor lungs after 2 h of brain death, under the assumption that there was no harmful impact on lung function until 5 h following brain death 22. However, other studies have shown that 6 h is the minimum elapsed time following brain death necessary for the analysis of inflammatory mediators 23.
TNF-α is the main inflammatory mediator after brain death. In addition to its direct participation in the inflammatory process, it is also responsible for the activation of apoptotic processes 24. Zhou HC et al. showed that carbon monoxide inhalation reduced the expression of TNF-α, IL-6, adhesion molecules, and pro-apoptotic pathways, improving PaO2/FiO2 and the base excess and acidosis generated by brain death 25. Our study showed that the administration of a corticosteroid 1 h after brain death can reduce the concentration of TNF-α, even after lung transplantation, thus demonstrating its benefit in this scenario.
Several studies have measured IL-1β levels in models of acute lung injury 26, brain death 27, and lung transplantation 28. High levels of IL-1β have previously been associated with acute lung injury and rejection 26. In our study, we observed a significant decrease in IL-1β in the transplanted lungs of brain-dead donors treated with methylprednisolone, reflecting its potential protective anti-inflammatory effect after lung reperfusion, which would consequently reduce ischemia-reperfusion injury.
Cytokines released after brain injury are likely crucial factors associated with the inflammatory activation that takes place in peripheral organs. Severe cerebral injury leads to the release of cytokines, such as TNF-α, IL-1β, and IL-6, from astrocytes and microglial cells 29. These cytokines cross the blood-brain barrier and reach peripheral organs and tissues 30, resulting in the expression of IL-1, IL-2, IL-6, TNF-α, and INF-Γ in these organs, as well as the lungs 31. An experimental study that performed cross circulation between controls and rats that underwent sudden brain death revealed an elevation of levels of lymphocytic and leukocyte inflammatory molecules 31.
Focal or generalized cerebral ischemia promotes the release of IL-1β and TNF-α in the brain 32, which induces the production of IL-6. It has been shown that patients in the intensive care unit for cerebral trauma had higher IL-6 levels in their jugular venous blood than their arterial blood 33. A clinical study showed that patients with stroke exhibited an elevated expression of IL-1β, IL-8, and IL-17 in peripheral mononuclear cells and concluded that cerebral ischemia stimulates the production of inflammatory cytokines in peripheral leukocytes 34.
After brain death, hemodynamic events occur concomitantly with systemic inflammatory events. This systemic inflammatory response has been associated with the production of cytokines by the brain. However, the precise role of these cytokines and whether the inflammatory response is due to central or peripheral factors are unclear 35.
In this study, the administration of methylprednisolone did not change hemodynamics and blood exchange prior to and after transplantation, indicating that its administration does not add any additional detrimental effect, as was previously shown by Pilla et al. 9. In contrast, Wigfield et al. showed that in a rat transplantation model, methylprednisolone treatment resulted in better graft function and less inflammatory activity after transplantation 8.
The present study has some limitations, and extrapolation of our results to clinical scenarios should be carefully performed. This study used a small, standardized animal model that involved the induction of brain death by a sudden increase in intracranial pressure. These conditions do not always match the clinical situation because intracranial pressure sometimes rises at a slow rate until brain death is established. Thus, a brain death model that employs a gradual rise of intracranial pressure may be more appropriate. Additionally, the interval between the establishment of brain death and lung harvest was shorter than is common in clinical practice. However, in our experimental model, we tried to reduce all of the confounding factors that were related to the time of preservation and that were detrimental to lung function due to long periods of ventilation after brain death.
We conclude that the administration of methylprednisolone after 60 min of brain death in donor rats reduces the inflammatory response after lung transplantation and has no effect on lipid peroxidation activity. Further studies with larger animals and models that better simulate the clinical scenarios, such as a longer period of brain death, a longer period prior to intervention, and a gradual increase in the intracranial pressure, are needed to confirm the beneficial effects of this treatment method. We suggest that the administration of methylprednisolone in potential multi-organ donors may be a useful strategy for reducing the dangerous effects of reperfusion after lung transplantation.
ACKNOWLEDGMENTSThis research was supported by grants from FIPE/HCPA (Hospital de Clínicas de Porto Alegre Institutional Research Fund) and National Counsel of Technological and Scientific Development (CNPq). We express our gratitude to American Journal Experts for proofreading this manuscript.
AUTHOR CONTRIBUTIONSAraújo LF conceived the study, collected the data, participated in the analysis of the samples, and drafted the manuscript. Holand AR, Forgiarini Jr LA, Forgiarini LF, Silva EF, Paludo AO, and Silva MB collected the data, participated in the analysis of the samples, and drafted the manuscript. Andrade CF conceived the study and drafted and approved the final version of the manuscript.
No potential conflict of interest was reported.