Experimental studies on sepsis have demonstrated that ethyl pyruvate is endowed with antioxidant and anti-inflammatory properties. This study aimed to investigate the effects of ethyl pyruvate on leukocyte-endothelial interactions in the mesenteric microcirculation in a live Escherichia coli-induced sepsis model in rats.
METHODS:Male Wistar rats were administered an intravenous suspension of E. coli bacteria or were subjected to a sham procedure. Three hours after bacterial infusion, the rats were randomized into the following groups: a control group without treatment, a group treated with lactated Ringer's solution (4 mL/kg, i.v.), and a group treated with lactated Ringer's solution (4 mL/kg, i.v.) plus ethyl pyruvate (50 mg/kg). At 24 h after bacterial infusion, leukocyte-endothelial interactions were investigated using intravital microscopy, and the expression of P-selectin and intercellular adhesion molecule-1 was evaluated via immunohistochemistry. White blood cell and platelet counts were also determined at baseline and 3 h and 24 h after E. coli inoculation.
RESULTS:The non-treated and lactated Ringer's solution-treated groups exhibited increases in the numbers of rolling leukocytes (∼2.5-fold increase), adherent cells (∼3.0-fold), and migrated cells (∼3.5-fold) compared with the sham group. In contrast, treatment with Ringer's ethyl pyruvate solution reduced the numbers of rolling, adherent and migrated leukocytes to the levels observed in the sham group. Additionally, the expression of P-selectin and intercellular adhesion molecule-1 was significantly increased on mesenteric microvessels in the non-treated group compared with the sham group (p<0.001). The expression of both adhesion molecules was reduced in the other groups, with ethyl pyruvate being more effective than lactated Ringer's solution. Infusion of bacteria caused significant leukopenia (3 h), followed by leukocytosis with granulocytosis (24 h). There was also an intense and progressive reduction in the number of platelets. However, no differences were observed after treatment with the different solutions.
CONCLUSIONS:The presented data suggest that ethyl pyruvate efficiently reduces the inflammatory response in the mesenteric microcirculation in an experimental model of sepsis induced by live E. coli and is associated, at least in part, with down-regulation of P-selectin and intercellular adhesion molecule-1.
Systemic inflammatory response syndrome (SIRS) and sepsis often affect the outcome of patients exposed to trauma, hemorrhage and major surgery, particularly among the elderly and immunocompromised 1,2. Even with therapeutic interventions aimed at better hemodynamic support and the development of new generations of antibiotics, sepsis and its complications are still responsible for high mortality rates in intensive care units 1,3–5.
Microcirculatory changes may herald the onset of multiple organ failure in critical conditions. These abnormalities include a characteristic heterogeneity of blood flow and decreased functional capillary density in patients with established severe sepsis 6–9, which are associated with tissue hypoxia 10 and organ dysfunction 11.
It has been shown that ethyl pyruvate improves survival and ameliorates organ dysfunction in animal models of ischemia-reperfusion injury, including hemorrhagic shock 12 and endotoxic shock 13,14 models. The anti-inflammatory and anti-oxidant properties of ethyl pyruvate observed in animal models of sepsis and septic shock 15,16 and in in vitro experiments 17 have demonstrated that ethyl pyruvate might be a promising therapeutic agent 18. It has specifically been shown that ethyl pyruvate ameliorates post-reperfusion deficits in the microvascular blood flow in the gut and liver as well as ileal mucosal hyperpermeability in mice subjected to mesenteric ischemia-reperfusion 19. Therefore, the present study aimed to investigate the effects of ethyl pyruvate, both in vivo and in situ, on the mesenteric microcirculation and the endothelial expression of P-selectin and intercellular adhesion molecule (ICAM)-1 in a sepsis model induced by live Escherichia coli in rats. This theme has been recently reviewed in a comprehensive manner 20,21.
MATERIALS AND METHODSAnimalsMale Wistar rats weighing 200–250 g at the beginning of the experiments were used. The animals were maintained at 23±2°C under a 12-h light/dark cycle and were allowed access to a standard rat chow pellet diet and water ad libitum. The experimental protocol was approved by the Ethical Committee of the Heart Institute (InCor) of the University of São Paulo Medical School and was performed according to the National Institutes of Health guidelines for the use of experimental animals.
Experimental protocolAnimals were anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg). A right cervical incision was performed, and the carotid artery and jugular vein were dissected and cannulated with polyethylene catheters (PE-50 coupled with PE-10). The catheters were filled with heparinized saline and exteriorized to the dorsum. All animals were then returned to their cages for recovery. The venous catheter was subsequently used for the injection of solutions, and the arterial catheter was used for monitoring the mean arterial blood pressure (MAP) and for collecting blood samples. On the day of the experiments, the rats were administered an intravenous suspension of E. coli bacteria (3−5×109 CFU/mL) or were subjected to a sham procedure. Three hours after bacterial infusion, the rats were randomized into the following groups: a control, non-treated (NT) group, a group treated with lactated Ringer's solution (LR group, 4 mL/kg), and a group treated with lactated Ringer's solution (4 mL/kg) plus ethyl pyruvate (REP group, 50 mg/kg). Commercially available LR solution (Baxter International Inc, São Paulo, SP, Brazil) was used as the control solution because of the increased solubility and stability of ethyl pyruvate when formulated in a calcium-containing balanced salt solution such as LR 22 and in order to preclude any effects of the electrolyte composition of the solutions on the observed differences. The ethyl pyruvate was purchased from Sigma Chemical Co (St Louis, MO, USA). The dose selected for this study was based on previously published studies 15,19. The direct MAP (mmHg) was recorded in unanesthetized rats by connecting the arterial catheter to a pressure transducer (Statham-Gould, P23 Db, Valley View, OH, USA) coupled to a multichannel recorder (PowerLab Multirecord, USA). Rectal temperature changes were simultaneously recorded. Arterial blood gases and electrolytes, blood lactate, and hematocrit analyses were also performed on blood samples obtained from the carotid artery at baseline (0 h) and 3 h and 24 h after E. coli inoculation using a gas analyzer (Radiometer ABL 555, Radiometer Medical, Copenhagen, Denmark). Moreover, white blood cell (WBC) and platelet counts were determined in blood samples obtained from the cut tip of the tail at baseline (0 h) and 3 h and 24 h after E. coli inoculation, and total cell counts were determined using a hemocytometer. Differential cell counts were carried out on stained films using oil immersion microscopy; a total of 100 cells were counted and classified on the basis of normal morphological criteria. Intravital microscopy of the mesenteric microcirculation and immunohistochemistry were additionally performed to detect P-selectin and ICAM-1 24 h after bacterial infusion.
Bacterial inoculumThe inoculum of bacteria was obtained from a culture of E. coli previously isolated from the lymph nodes of Wistar rats subjected to small bowel obstruction 23. Culture samples were plated on agar (TSA Tryptic Soy Agar, DIFCO®) and incubated for 18 h at 37°C. Two hours before inoculation, bacterial samples were suspended in saline (0.9% NaCl) to achieve an optical density of 2.0 at a wavelength of 625 nm. This bacterial suspension results in approximately 50% mortality within 7 days.
Intravital microscopy of the mesenteric microcirculationAnimals were anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg). After making an abdominal midline incision, the distal ileum and its accompanying mesentery were exposed for in vivo microscopic examination of the microcirculation, as previously described 24,25. The animals were placed on a specially designed stage warmed by circulating water maintained at 37°C. This stage has a transparent platform on which the tissue to be transilluminated is placed. The mesentery was continuously perfused throughout the study period with warmed (37°C) Krebs-Henseleit solution (113 NaCl, 4.7 KCl, 2.5 CaCl2·2 H2O, 25 NaHCO3, 1.1 MgSO4, 1.1 KH2PO4 and 5 mmol/L glucose, pH 7.20−7.40) that was saturated with a mixture of gases (95% N2 and 5% CO2). This procedure kept the microcirculatory characteristics unchanged throughout the intravital microscopic analysis. The mesenteric microcirculation was assessed after 10 min of stabilization, and 3–5 postcapillary venules (diameter 20–25 µm) were selected in each animal. A charge-coupled device color camera (TK-C1380U, JVC Co, Tokyo, Japan) was incorporated into a trinocular microscope (Axioplan 2, Carl Zeiss Co, München-Hallbergmoos, Germany) to facilitate observation of the magnified image (425x) on a microcomputer monitor. Analyses of leukocyte-endothelial interactions were performed online using image-processing computer software (Axiovision 4.1, Carl Zeiss Co) with an incorporated modulus of interactive measurements and time lapse. The images were stored, enabling offline playback analysis. Rolling leukocytes were defined as WBCs that moved at a velocity that was significantly slower than that of erythrocytes in a given microvessel, and the number of rolling leukocytes is presented as the mean number of cells passing a designated line perpendicular to the venular axis per 3 min. In contrast, a leukocyte was considered to be adherent to the venular endothelium if it remained stationary for more than 30 s, and adherent cells were counted in a 100 µm segment of the vessel. The number of leukocytes accumulating in the connective tissue, adjacent to the studied postcapillary venule, was determined in a standard area of 5,000 μm2.
Immunohistochemistry for P-selectin and ICAM-1Animals were anesthetized, as described above, and exsanguinated via abdominal aorta puncture. The mesentery was then removed and immediately frozen in a liquid nitrogen-hexane solution. Serial 8-μm-thick cryosections were placed on organosilane-coated slides (Dako, Glostrup, Denmark) and fixed in cold acetone for 10 min prior to direct immunohistochemical assays. Non-specific sites were blocked by incubation of the sections with Tris-buffered saline-Tween20 (TBST) containing 3% bovine serum albumin for 1 h at room temperature. The sections were then incubated overnight in a humidified chamber (4°C) with either a monoclonal anti-human P-selectin antibody (CD62P; R&D Systems Inc, Minneapolis, MN, USA) or a monoclonal anti-rat ICAM-1 antibody (CD54; Cedarlane Laboratories, Ontario, Canada). Both antibodies were diluted 1:50 in TBST containing 1% bovine serum albumin. Endogenous peroxidase was also blocked (Dual Enzyme Block, Dako) by incubation of the sections for 15 min at room temperature. After washing the slides with TBST, the sections were incubated with secondary antibodies (ADVANCE™ HRP Link, Dako) for 30 min at room temperature, rinsed in TBST, and then incubated with HRP-conjugated antibodies (ADVANCE™ HRP Enzyme, Dako) for 30 min at room temperature. After further washes, the samples were developed with the HRP substrate 3-amino-9-ethylcarbazole (AEC; Vector Laboratories, Burlingame, CA, USA) for 10 min and counterstained with Mayer's hematoxylin. The background reaction was determined in sections incubated in the absence of primary antibody (negative control). The slides were mounted, and images were acquired with a DS-Ri1 digital camera (Nikon, Tokyo, Japan) connected to a microscope (Nikon) using NIS-Elements BR software (Nikon). The results are presented as objects×103/μm2.
Statistical analysisThe data are presented as the mean ± SEM. Statistical comparisons between groups were performed using ANOVA for repeated measures and one-way ANOVA, and differences between groups were tested with the Tukey-Kramer multiple-comparisons test. A value of p<0.05 was considered statistically significant.
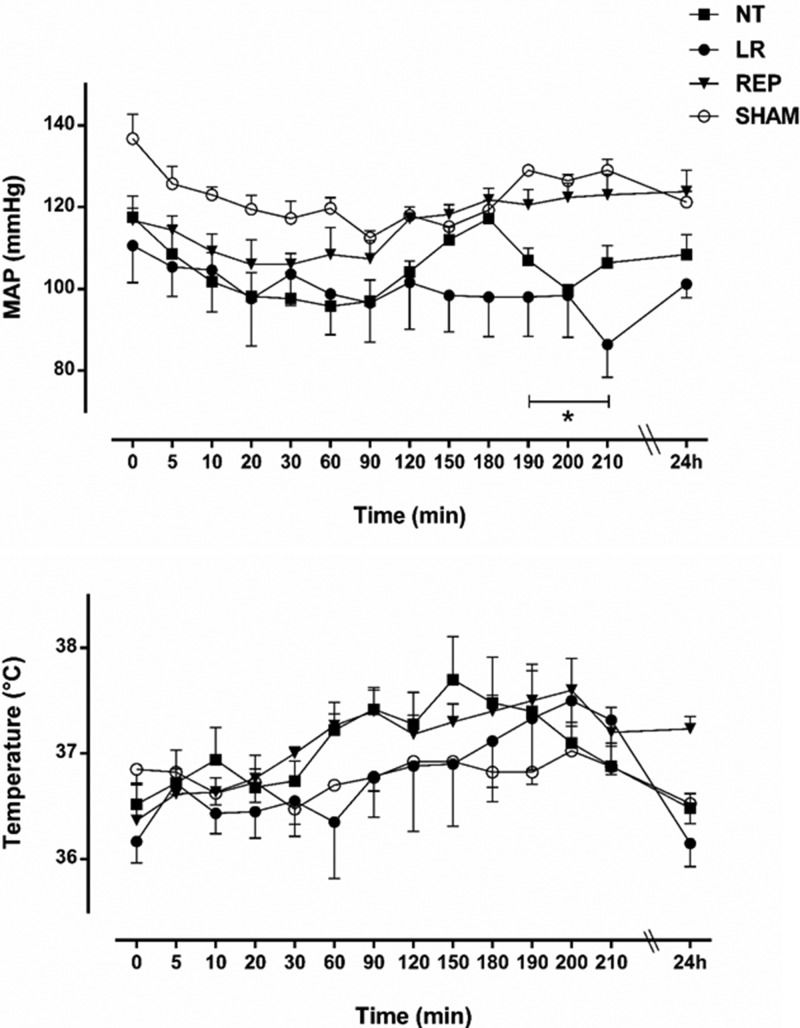
RESULTSEffects of the treatments on systemic variablesAs illustrated in Figure 1, the effects of the LR and REP treatments on the MAP and body temperature were assessed for 30 min following the initiation of E. coli infusion (180 min) and at 24 h thereafter. Bacteremia caused a slight reduction in the MAP, which returned to baseline only in the REP group. Meanwhile, a progressive increase in body temperature was observed in all groups after bacterial infusion, although neither the LR nor the REP treatment modified body temperature during the 30 min following E. coli infusion. A reduction in the levels of bicarbonate and base excess was also observed in the first 3 h after bacterial infusion and was maintained at 24 h. However, there were no differences between groups (data not shown). Compared with the sham group, which presented 100% survival, the NT group exhibited 40% mortality (9/23), and in the LR and the REP treatment groups, the mortality rates were reduced to 26% (5/19) and 25% (4/16), respectively.
Effects of lactated Ringer's (LR, 4 mL/kg, n=7) and Ringer's ethyl pyruvate (REP, 4 mL/kg LR + 50 mg/kg ethyl pyruvate, n=6) solutions compared with no treatment (NT, n=7) and sham operation (SHAM, n=5) on the mean arterial pressure (mmHg) and body temperature (°C) during live E. coli-induced bacteremia. The treatments were initiated at 180 min. The data are presented as the mean ± SEM. *p<0.05 for LR and NT versus SHAM.
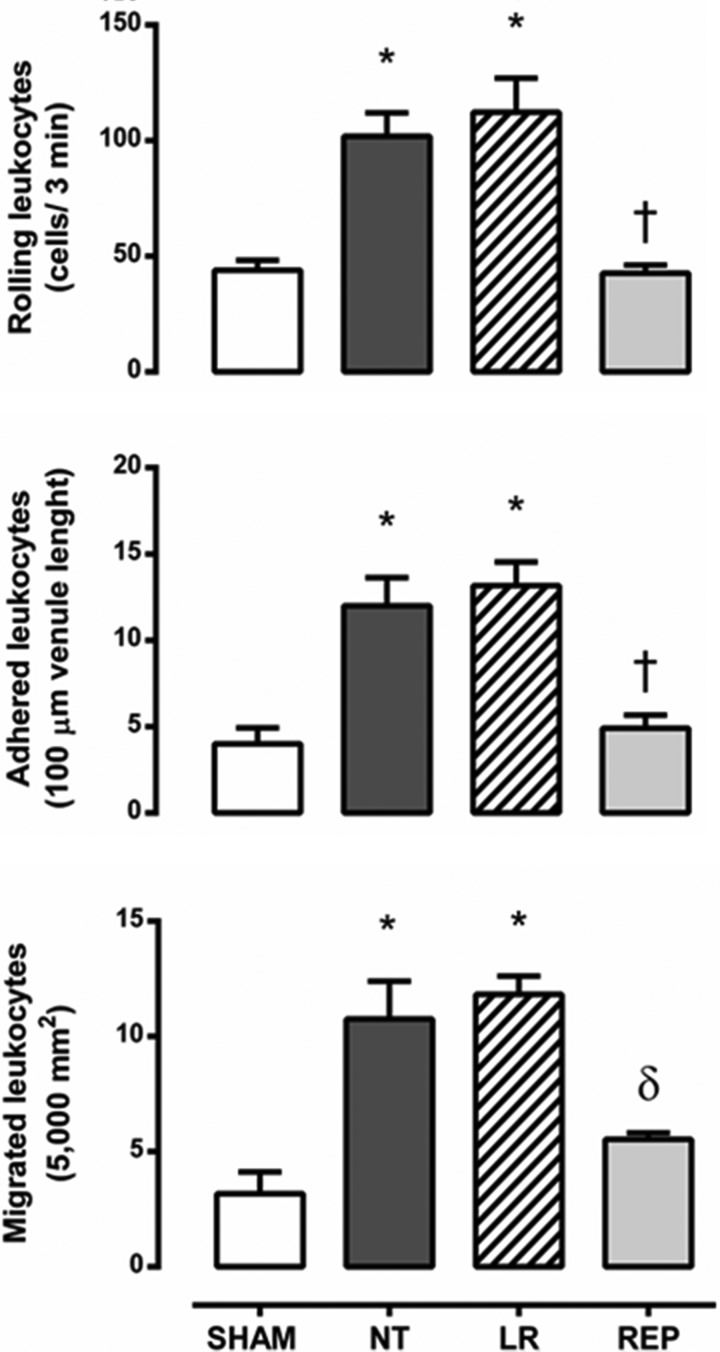
For observation of the mesenteric microcirculation, single and unbranched postcapillary venules were selected; their diameters ranged from 20–25 μm in all groups. The observed leukocyte-endothelial interactions, assessed after 24 h of bacteremia, showed that treatment of the animals with REP solution significantly reduced the number of rolling leukocytes to the level observed in the sham group, whereas there was an increased number (∼2.5-fold increase) of rolling cells in the NT and LR groups. Similarly, REP solution reduced the numbers of adherent and migrated leukocytes to the levels attained in the sham group, whereas in both the NT and the LR groups, there were increased numbers of adherent leukocytes (∼3-fold increase) and migrated cells (∼3.5-fold) compared with numbers in the sham group (Figure 2). Representative photomicrographs are shown in Figure 3. Analyses of WBC counts showed that the infusion of bacteria caused leukopenia at 3 h due to a reduction in the number of lymphocytes and monocytes, followed by significant leukocytosis with granulocytosis at 24 h. Furthermore, there was an intense and progressive reduction in the number of platelets. However, no differences were observed after treatment of the animals with either the LR or the REP solution. Meanwhile, sham-operated rats exhibited transient leukocytosis with granulocytosis associated with the surgical procedure. The results are summarized in Table 1.
The number of rolling (3 min), adherent (100 μm venule length) and migrated (5,000 μm2) leukocytes in the rat mesenteric microcirculation 24 h after intravenous injection of E. coli. SHAM, sham-operated rats (n=5); NT, non-treated rats (n=7); LR, lactated Ringer's solution (4 mL/kg)-treated rats (n=7); REP (4 mL/kg LR + 50 mg/kg ethyl pyruvate)-treated rats (n=6). The data are presented as the mean ± SEM. *p<0.001 versus SHAM, †p<0.001 and ?p<0.01 versus NT and LR.
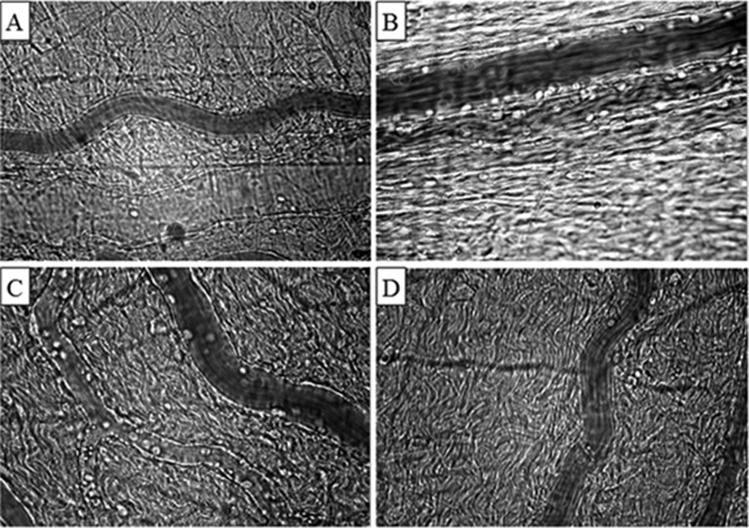
Representative photomicrographs of the mesenteric microcirculation of a sham-operated rat (A); a control, non-treated rat (B); a lactated Ringer's solution-treated rat (C); and a Ringer's ethyl pyruvate solution-treated rat (D). Increased leukocyte-endothelial interactions were observed in the non-treated (B) and lactated Ringer's solution-treated (C) rats compared with the Ringer's ethyl pyruvate solution-treated (D) and sham (A) rats. Final magnification, 425X.
White blood cell and platelet counts
| Group | Baseline | 3 h | 24 h |
|---|---|---|---|
| SHAM | |||
| WBCs/mm3 | 9,720±1,028 | 21,460±2,280* | 18,575±605* |
| Granulocytes/mm3 | 2,800±195 | 16,340±1,815† | 8,700±755§ |
| Lymphocytes/mm3 | 6,660±824 | 4,640±490 | 9,075±517 |
| Monocytes/mm3 | 260±21 | 480±73 | 800±82† |
| Platelets x103/mm3 | 858±26 | 628±32* | 817±53 |
| NT | |||
| WBCs/mm3 | 13,430±366 | 7,280±405 | 5,864±2,434† |
| Granulocytes/mm3 | 6,250±256 | 4,808±235 | 9,758±2,291† |
| Lymphocytes/mm3 | 6,658±307 | 2,306±234† | 5,762±827 |
| Monocytes/mm3 | 522±29 | 166±17† | 360±44§ |
| Platelets x103/mm3 | 884±57 | 336±27† | 74±11† |
| LR | |||
| WBCs/mm3 | 11,647±928 | 6,940±336§ | 22,860±1,319† |
| Granulocytes/mm3 | 4,834±293 | 4,840±522 | 17,507±1,016† |
| Lymphocytes/mm3 | 6,300±732 | 1,947±248† | 4,766±488 |
| Monocytes/mm3 | 513±73 | 153±6§ | 586±101 |
| Platelets x103/mm3 | 800±53 | 271±33† | 118±15† |
| REP | |||
| WBCs/mm3 | 12,405±665 | 6,501±695 | 6,906±4,790§ |
| Granulocytes/mm3 | 5,203±283 | 4,381±561 | 0,986±4,328* |
| Lymphocytes/mm3 | 6,733±380 | 1,893±297† | 5,413±680 |
| Monocytes/mm3 | 468±68 | 226±79 | 506±75 |
| Platelets x103/mm3 | 806±50 | 410±58† | 78±19† |
SHAM, sham-operated, n=5; NT, non-treated, n=7; LR, lactated Ringer's solution, n=7; REP, Ringer's ethyl pyruvate solution, n=6. The data are presented as the mean ± SEM. †p<0.001; *p<0.01; §p<0.05 versus baseline values.
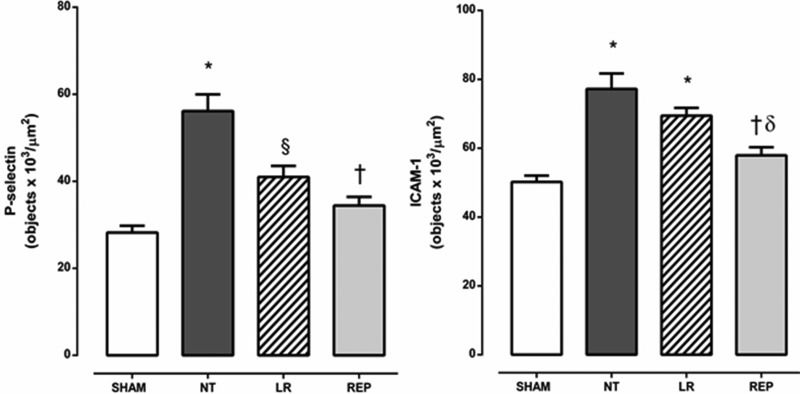
As illustrated in Figure 4, the expression of P-selectin and ICAM-1 on mesenteric microvessels was significantly increased in the NT group compared with the sham group. Treatment of the animals with REP solution reduced the expression of both adhesion molecules (P-selectin and ICAM-1) to the levels attained in the sham group. In contrast, treatment with LR partially reduced P-selectin expression but did not modify ICAM-1 expression on mesenteric microvessels.
Quantitative evaluation of P-selectin and ICAM-1 expression on rat mesenteric microvessels 24 h after intravenous injection of E. coli. SHAM, sham-operated rats; NT, non-treated rats; LR, lactated Ringer's solution (4 mL/kg)-treated rats; REP (4 mL/Kg LR + 50 mg/kg ethyl pyruvate)-treated rats. The presented values are the mean ± SEM for 3 samples/rat, with 3 rats/group. The analyses were performed using NIS-Elements BR software (Nikon). The results are presented as objectsx103/μm2. *p<0.001 versus SHAM, §p<0.01 versus SHAM and NT, †p<0.001 versus NT, ?p<0.01 versus LR.
The data presented herein demonstrated that early administration of ethyl pyruvate during bacteremia induced by live E. coli efficiently reduces the number of rolling, adherent and migrated leukocytes in the mesenteric microcirculation and down-regulates the endothelial expression of P-selectin and ICAM-1.
In this experimental model, the development of sepsis was triggered by live E. coli bacteria obtained from the mesenteric lymph nodes of rats subjected to strangulated small bowel obstruction 23 in an attempt to mimic the scenario of bacterial translocation that might occur in clinical settings. In small animals, low doses of E. coli have been associated with minimal early physiological changes, whereas higher doses result in immediate cardiovascular collapse and early death 26. In the present study, a bacterial inoculum that results in approximately 50% mortality within 7 days was established. As demonstrated herein, the mortality rate was 40% in the NT group. At the onset, the clinical manifestations were characterized by piloerection and decreased activity, followed by a slight reduction in the MAP and an increased heart rate. During the course of bacteremia, our findings comprised positive criteria for the diagnosis of sepsis and SIRS, such as elevated temperatures, reduced PCO2 due to hyperventilation, leukopenia followed by leukocytosis, and significant thrombocytopenia at the end of the experiments. Substantial changes in leukocyte-endothelial interactions were also observed via intra-vital microscopy at the same time.
In cases of sepsis, leukocyte and endothelial cell activation is a well-known primary host immune reaction against bacterial invasion 27. In particular, leukocytes must be activated before they can attach to the microvessel wall and migrate into the surrounding tissue. This process is mediated by a variety of glycoproteins expressed on the surface of leukocytes and endothelial cells, resulting in increased rolling and sticking of leukocytes to postcapillary venules. Leukocyte rolling along the walls of microvessels is specifically mediated by the selectin family of adhesion molecules, such as P-selectin 28, after which the leukocytes become firmly adhered to the vascular wall through the interaction between ICAM-1 on endothelial cells and β2 integrins (CD11/CD18) on leukocytes 29.
The data presented herein showed that the administration of ethyl pyruvate was effective in reducing inflammation, as demonstrated by decreased leukocyte-endothelial interactions and down-regulated expression of P-selectin and ICAM-1 on mesenteric microvessels in vivo. Indeed, in vitro studies in human umbilical vein endothelial cells (HUVECs) 30 and a human lung epithelial cell line 31 demonstrated that ethyl pyruvate displays anti-inflammatory activity. It has also been shown that ethyl pyruvate reduces the adhesion of neutrophils to activated HUVECS; the generation of interleukin (IL)-8; and the expression of ICAM-1, vascular cell adhesion molecule (VCAM)-1, and E-selectin on the surface of endothelial cells 30. Furthermore, exposure of lung epithelial (A549) cells to ethyl pyruvate in vitro inhibits generation of the inflammatory cytokines IL-8 and granulocyte colony-stimulating factor and reduces the expression of ICAM-1 and VCAM-1 and the adhesiveness of human neutrophils to lung epithelial cells 31.
The beneficial effects of ethyl pyruvate on leukocyte-endothelial interactions are not related to differences in the WBC count during the course of bacteremia, as similar leukocytosis with granulocytosis was observed in NT as well as LR-treated rats. Furthermore, rats treated with LR solution exhibited a partial reduction in P-selectin expression. Nevertheless, ICAM-1 expression and microcirculatory injury did not change with LR treatment. Additionally, ethyl pyruvate did not modify the progressive reduction in the number of platelets, in contrast to reported findings, which showed that lipopolysaccharide-induced disseminated intravascular coagulation in rats benefits from treatment with ethyl pyruvate 32.
In conclusion, the data presented herein suggest that early treatment of sepsis with ethyl pyruvate efficiently reduces microcirculatory dysfunctions and is associated with down-regulation of P-selectin and ICAM-1 expression.
*Presented at the Meeting of the British Microcirculation Society and the Microcirculatory Society, July 4-6, 2012, Oxford, UK.
AUTHOR CONTRIBUTIONSGuarda IF participated in the research design, the performance of the research, the data analysis, and the writing of the paper. Correia CJ provided surgical and intravital microscopic support. Breithaupt-Faloppa AC provided help with the immunohistochemistry. Ferreira SG participated in the performance of the research. Moreno AC provided help with preparation of the bacterial inoculum. Martinez MB supervised the preparation of the microbiological cultures. Rocha-e-Silva M participated in the research design and the data analysis. Sannomiya P participated in the research design, the data analysis, and the writing of the paper.
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).