Poor sleep quality is common among patients following cardiopulmonary artery bypass graft surgery. Pain, stress, anxiety and poor sleep quality may be improved by massage therapy.
OBJECTIVE:This study evaluated whether massage therapy is an effective technique for improving sleep quality in patients following cardiopulmonary artery bypass graft surgery.
METHOD:Participants included cardiopulmonary artery bypass graft surgery patients who were randomized into a control group and a massage therapy group following discharge from the intensive care unit (Day 0), during the postoperative period. The control group and the massage therapy group comprised participants who were subjected to three nights without massage and three nights with massage therapy, respectively. The patients were evaluated on the following mornings (i.e., Day 1 to Day 3) using a visual analogue scale for pain in the chest, back and shoulders, in addition to fatigue and sleep. Participants kept a sleep diary during the study period.
RESULTS:Fifty-seven cardiopulmonary artery bypass graft surgery patients were enrolled in the study during the preoperative period, 17 of whom were excluded due to postoperative complications. The remaining 40 participants (male: 67.5%, age: 61.9 years ± 8.9 years, body mass index: 27.2 kg/m2 ± 3.7 kg/m2) were randomized into control (n = 20) and massage therapy (n = 20) groups. Pain in the chest, shoulders, and back decreased significantly in both groups from Day 1 to Day 3. The participants in the massage therapy group had fewer complaints of fatigue on Day 1 (p = 0.006) and Day 2 (p = 0.028) in addition, they reported a more effective sleep during all three days (p = 0.019) when compared with the participants in the control group.
CONCLUSION:Massage therapy is an effective technique for improving patient recovery from cardiopulmonary artery bypass graft surgery because it reduces fatigue and improves sleep.
Pain, stress, anxiety, and sleep disorders are common after surgery. All of these factors can compromise treatment and quality of life following surgery.1 Evidence indicates that patients' quality of sleep after heart surgery is frequently poor, particularly during the postoperative period, and that patients experience high levels of sleep disruption, irregular sleep cycles, and reductions in slow-wave sleep.2 Poor quality of sleep in the postoperative period may be due to several factors, including pain from surgical incision, presence of a thoracic drain, pain caused by prolonged time in bed, and high anxiety levels.1 In addition, muscle pain, particularly in the neck, shoulders and back, may make it difficult for patients to breath, cough, move, and sleep.1
Massage therapy (MT) is a technique that promotes the manual mobilization of several structures from both muscle and subcutaneous tissue, by applying mechanical force to tissues. This mobilization improves lymph movement and venous return; reduces swelling; and mobilizes muscle fibers, tendons and skin. Thus, MT may be used to promote muscle relaxation and to reduce pain, stress and anxiety,3 which help patients improve their quality of sleep and speed recover. In addition, MT may enhance patient mobility and recovery from surgery, which allows patients to perform daily activities3 and take part in physiotherapy treatment and rehabilitation. The European Respiratory Society and the European Society of Intensive Care Medicine recognize that MT may improve quality of sleep.4 Therefore, in this study, we evaluated whether MT is an effective method for improving quality of sleep in patients following coronary artery bypass graft (CABG) surgery.
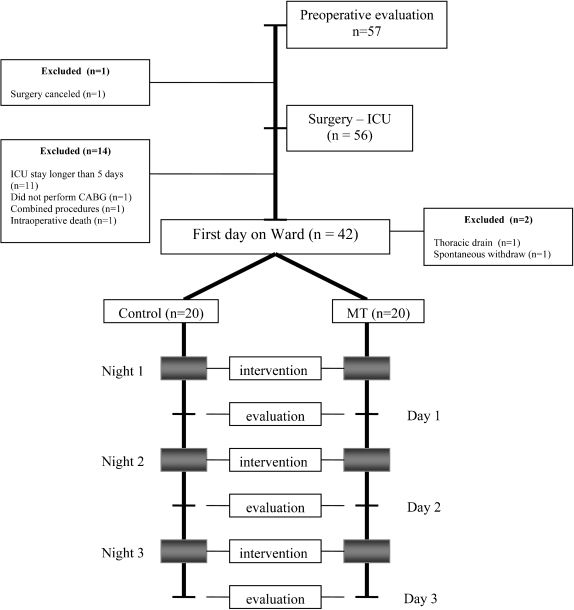
MATERIALS AND METHODSPatientsWe evaluated patients who were waiting for elective CABG surgery between May 2008 and January 2009 at the Heart Institute (InCor), University of São Paulo School of Medicine, São Paulo, Brazil. Inclusion criteria included patients of both genders between 40 and 80 years of age. We excluded patients who had a body mass index (BMI) ≥ 35 kg/m2, history of regular alcohol consumption, chronic use of hypnotics, previous diagnosis of sleep disorders, surgery other than CABG performed within the last 24 months, or an illiteracy. We also excluded patients who had combined valve and CABG surgery, a thoracic drain in their return to the ward, or an intensive care unit (ICU) stay longer than 5 days in the postoperative period. All patients provided written informed consent, and the local ethics committee approved our protocol.
Data CollectionPreoperative evaluationThe preoperative clinical assessment consisted of obtaining information regarding the patients' demographics and personal characteristics. We aimed to characterize sleep, pain and fatigue complaints before surgery by applying the following questionnaires:
Epworth Sleepiness Scale (ESS)The ESS was used to evaluate excessive daytime sleepiness in participants. The participants rated the probability of dozing, on a scale of 0 to 3, in 8 different situations. A score over 10 indicated the presence of excessive daytime sleepiness.5 A Portuguese version of this scale has been previously validated for use in Brazil.6
The Pittsburgh Sleep Quality IndexThis index was used to evaluate the quality of sleep of the participants, according to seven components, during the thirty days leading up to the study. The participants' final scores were interpreted as follows: 0-4 indicated good sleep quality, 5 - 10 indicated poor sleep quality, over 10 indicated the presence of a sleep disorder.7 A Portuguese version of this questionnaire has been previously validated for use in Brazil.8
Berlin QuestionnaireThis questionnaire was used to classify participants at low- and high-risk for obstructive sleep apnea (OSA), based on the following three symptom categories: (1) snoring, (2) tiredness, and (3) clinical characteristics. In the first category, high risk was defined as having persistent symptoms (i.e., 3 to 4 times per week) for two or more of the questions regarding snoring. In the second category, high risk was defined as having either persistent (i.e., 3 to 4 times per week) wake time sleepiness, persistent drowsiness while driving, or both. In the third category, high risk was defined as a history of high blood pressure or a BMI >30 kg/m2. To be considered at high risk for OSA, participants had to be at high risk for at least two symptom categories. Those patients who were at high risk for only one symptom category or denied having persistent symptoms were considered at low-risk for OSA.9
Visual Analogue Scale (VAS) for pain and fatigueThe VAS was used to evaluate pain and fatigue in participants. Participants indicated the intensity of pain and fatigue on a scale of 0 to 10, where 0 and 10 corresponded to minimum and maximum, respectively.10
Postoperative protocol and evaluationFollowing discharge from the ICU to the ward during the postoperative period (Day 0), the participants were randomized into control and MT groups. The control participants sat in comfortable chairs for three consecutive nights and were not subjected to MT. MT was performed by the same physiotherapist during the protocol and consisted of neck, shoulder and back massages. The massages were initiated with light compression by the inner regions of the fingers and progressed to hard compression. Manual kneading, friction (i.e., digital compression with the thumb) on trigger points, cervical traction, and mobilization in all planes (e.g., the front, back, and sides) followed. The massage was finished with light manual compression. This intervention was performed around 7 PM, 2-3 hours before sleep. Trigger points were areas that triggered pain during the massages. Over the duration of the study, participants completed a sleep diary in which they recorded the times at which they went to sleep, woke up, and took daytime naps. Both, the MT and control participants were evaluated for their pain and fatigue levels and their sleep quality each morning following the previous night Pain and fatigue were evaluated by the VAS, as described above, during the preoperative period. Sleep quality was evaluated by the VAS of sleep, which has been previously adapted and validated in Portuguese.11 The VAS of sleep consisted of 16 items from 3 categories as following: sleep disorder scale, effectiveness scale and supplementation scale. The sleep disorder scale looked at seven items and evaluated the degree of sleep impairment caused by fragmentation and late sleep onset. Scores ranged from 0 to 70, and higher values indicated a worse case of sleep disorder. The effectiveness scale looked at five items and measured the degree of sleep effectiveness. Scores ranged from 0 to 50, and higher values indicated more effective sleep. The supplementation scale looked at four items and measured the degree to which additional periods of sleep (daily naps) were necessary to supplement the main period of sleep. Scores ranged from 0 to 40, and higher values indicated that additional naps were necessary during the day.12
Data AnalysisThe Student t, Mann-Whitney, or chi-squared tests, when appropriate, were used to compare the control and MT groups at baseline. The two-way repeated measures analysis of variance (ANOVA) was used to compare the control and MT groups. A p≤0.05 was considered significant. The data were analyzed with SPSS 15.0 software.
RESULTSA total of 57 CABG surgery patients were invited to participate in the study during the preoperative period. Seventeen patients were excluded for the following reasons: ICU stay was longer than 5 days (n = 11), spontaneous withdrawal from the study (n = 1), thoracic drain put in place upon return to the ward (n = 1), combined surgical procedures (n = 2), cancellation of surgery (n = 1), and death during surgery (n = 1). The final study sample consisted of 40 patients who were randomized into the control (n = 20) and MT (n = 20) groups (Figure 1).
Demographic, clinical, and sleep characteristics of the participants are presented in Table 1. No significant differences existed in the anthropometric characteristics between the groups, except for BMI, which was significantly higher in the control group. During the preoperative period, 30% of patients from the control group and 35% from the MT group had complaints of pain in the back and shoulders. The pain intensity was mild in both groups (p = 0.739). Complaints of fatigue were few among both groups (averaging 1.65 ± 2.55 for control and 1.35 ± 1.45 for MT group, p = 0.152). There were no significant differences between the control and the MT groups regarding the duration of the bypass (69.5 min ± 50.7 min and 58.6 min ± 45.7 min, respectively, p = 0.29), time on mechanical ventilation in the ICU (6.8 h ± 2.4 h and 7.2 h ± 3.1 h, respectively, p = 0.82), length of ICU stay (69.2 h ± 27.6 h and 66.8 h ± 31.9 h, respectively, p = 0.51), and length of hospital stay (7.55 d ± 1.23 d and 7.75 d ± 2.38 d, respectively, p = 0.923).
Demographic and clinical characteristics of participants from the control and MT groups.
| Control | MT | P | |
|---|---|---|---|
| (n = 20) | (n = 20) | ||
| Male, n (%) | 13 (65) | 14 (70) | 1.00 |
| Age, yrs | 60 ± 8 | 63 ± 9 | 0.27 |
| BMI, kg/m2 | 28.5 ± 4.1 | 25.9 ± 2.8 | 0.02∗ |
| Diabetes, n (%) | 11 (55) | 11 (55) | 1.00 |
| Hypertension, n (%) | 16 (80) | 16 (80) | 1.00 |
| Dyslipidemia, n (%) | 13 (65) | 11 (55) | 0.52 |
| Insomnia, n (%) | 1 (5) | 4 (20) | 0.34 |
| Epworth | 10 ± 5 | 11 ± 6 | 0.61 |
| Pittsburgh | 5 ± 2 | 6 ± 4 | 0.32 |
| High risk for OSA, n (%) | 13 (65) | 8 (40) | 0.20 |
Values are presented as either means and standard deviations or percentages. BMI: body mass index; Epworth: Epworth sleepiness scale, Pittsburgh: The Pittsburgh Sleep Quality Index; high risk of Obstructive Sleep Apnea (OSA) determined by Berlin Questionnaire. ∗(p ≤ 0.05)
The total sleep time of participants during the study period was obtained from their sleep diaries and was similar between groups, with averages per night of 383 min ± 158 min and 385 min ± 116 min in the control and the MT groups, respectively (p = 0.536). Participants in both groups received similar quantities of dipyrone and tramadol as analgesics during the study period. Hypnotics were not used on any of the participants.
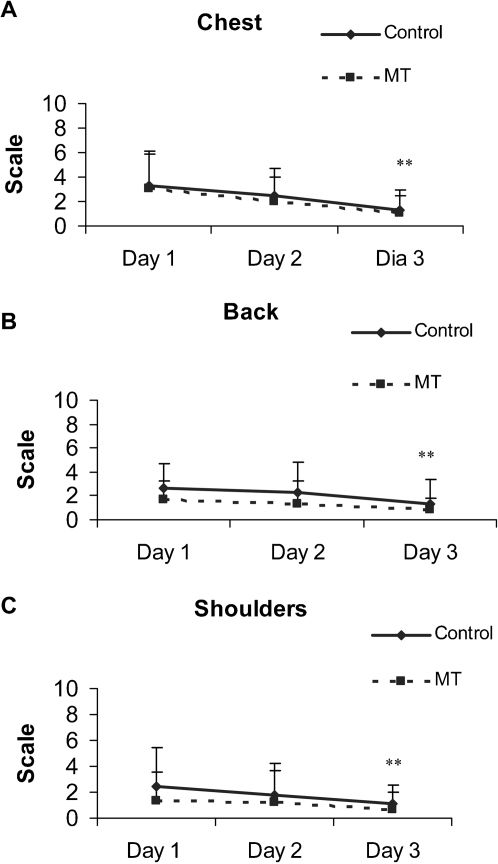
VAS scores for pain in the chest, back and shoulders during the postoperative period for each group are presented in Figures 2 A, B, and C. There was a significant decrease in the complaints of pain in the chest (p<0.001), back (p = 0.007) and shoulder (p = 0.036) from Day 1 to Day 3 in both groups.
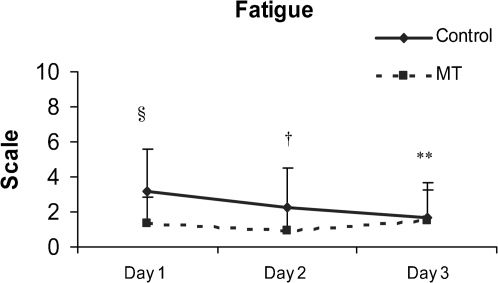
Complaints of fatigue among participants were different between the two groups (Figure 3). The control group had high fatigue scores on Day 1, which decreased over the duration of the study (p = 0.022). In contrast, the MT group had lower fatigue scores on Day 1 (p = 0.006) and Day 2 (p = 0.028) than the control group.
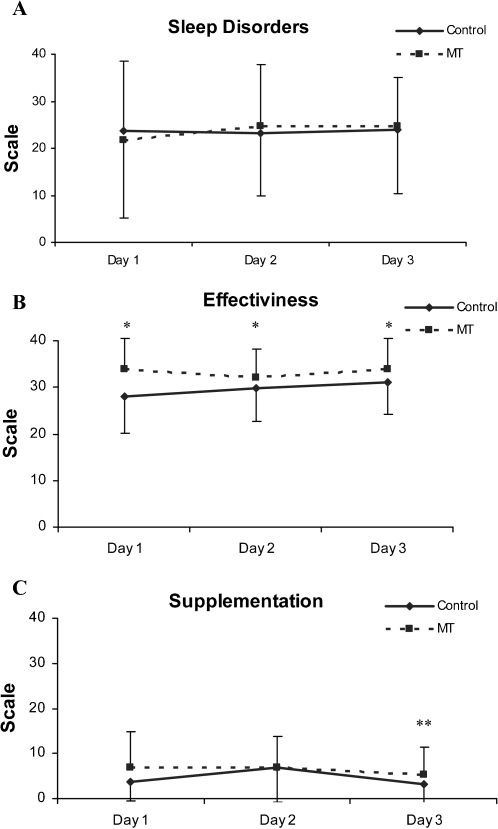
VAS scores for sleep characteristics exhibited by participants during the study period are presented in Table 2 and Figures 4 A, B, and C. The sleep disorder scale scores showed no significant intragroup (p = 0.936) or intergroup (p = 0.489) differences. Effectiveness scale scores were higher for the MT group than the control group (p = 0.019) during all the study period. Supplementation scale scores decreased (indicating less need for napping) on the third day in both groups (p = 0.031).
VAS of sleep scores in the control and MT groups during the study period.
| Control (n = 20) | MT (n = 20) | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | |
| Sleep disorders | 27.3 ± 14.7 | 26.1 ± 14.8 | 24.0 ± 11.1 | 21.8 ± 16.7 | 24.8 ± 15.0 | 24.8 ± 14.4 |
| Effectiveness | 28.0 ± 7.9 | 29.8 ± 7.1 | 31.0 ± 6.8 | 33.8 ± 6.6∗ | 32.0 ± 6.3∗ | 33.8 ± 6.6∗ |
| Supplementation | 3.7 ± 4.1 | 6.9 ± 7.8 | 3.2 ± 4.4∗∗ | 6.8 ± 8.1 | 6.9 ± 6.8 | 5.2 ± 6.3∗∗ |
Values are presented as means (±SD). ∗ Intergroup comparison between Day 1, Day 2, and Day 3; ∗∗ intragroup comparison between Day 2 and Day 3 (p ≤ 0.05).
This study evaluated the effects of MT in patients during the postoperative period after CABG surgery. The results indicate that MT improved the comfort level of the MT group participants in comparison with those patients in the control group. Recovery from fatigue was significantly faster in the MT group, reaching statistically significant differences by Day 1 and Day 2. Sleep effectiveness was also significantly higher in the MT group participants during all the study period. In addition, the results indicate that the beneficial effects of MT were not mediated by reducing pain because the number of complaints about pain was similar in both the control and MT groups.
We found that several participants had complaints of daily somnolence and poor quality of sleep during the preoperative period. This observation is consistent with previous findings that showed that poor sleep is common in patients awaiting surgical procedures.2 This poor quality of sleep is likely caused by pain, stress, and anxiety.13
In addition, patients with cardiovascular disease frequently have OSA, which is an independent risk factor for cardiovascular disease (e.g., hypertension, ischemic heart disease, atrial fibrillation, stroke, heart failure).14 OSA may contribute to poor sleep and excessive daytime somnolence during the preoperative period. In our sample, 55% of participants (n = 21) showed symptoms suggestive of OSA upon evaluation using the Berlin Questionnaire (Table 1). Thus, OSA may play a role in worsening sleep not only in the preoperative but also in the postoperative periods.
In addition to the symptoms present in the preoperative period, sleep fragmentation and deprivation are also commonly observed after surgery. Sleep deprivation per se is a stress condition, which causes the inappropriate activation of the hypothalamic-pituitary-adrenal axis. This activation results in an increase in cortisol release,15 the worsening of the immune system, and a predisposition to inflammation and infection.16 Furthermore, sleep deprivation may increase sympathetic nervous system activity and blood pressure.16-18 All of these factors may impact patient recovery. In our study, we observed that all participants reported having a certain degree of sleep disorder following surgery and a necessity for daily naps, both of which significantly decreased from Day 2 to Day 3. MT promoted improvement in sleep effectiveness (Figure 4B), indicating that it had a positive influence on sleep. Similar to our findings, Tsay and colleagues (2003)19 found that MT improved sleep quality in patients with end-stage renal disease. Field and colleagues (2002)20 observed that MT decreased anxiety and depressed mood and increased the number of sleep hours in patients with fibromyalgia. Thus, previous literature supports our finding that MT improves sleep and recovery in patients following surgery.
MT promoted a faster decrease in complaints of fatigue in the MT group participants than in those of the control group, reaching statistical significance by Day 1 and Day 2 (Figure 2). A previous study has shown that MT promotes a significant decrease in cortisol levels from the baseline (averaging 31%) and increase in active neurotransmitters, such as serotonin (averaging 28%) and dopamine (averaging 31%).21 MT may also promote the following: parasympathetic activation,3,22 which causes reductions in heart rate, blood pressure, and breathing; increases in the release of hormones (e.g., endorphins);3 and decreases in stress levels.22 For example, Field and colleagues (2002)20 found reductions in substance P, which is related to pain complaints, in patients with fibromyalgia who received MT. Such positive changes in conjunction with the beneficial effects of MT on sleep quality may play a role in reducing the effects of stress caused by disease or surgery21 and feelings of fatigue.
The beneficial effects of MT on pain have been previously described. Field and colleagues (2002)20 evaluated the effects of MT and relaxation therapy in a group of patients with fibromyalgia. In their study, a decrease in complaints of pain among participants assigned to the MT group was associated with a reduction in substance P. In a multi-center randomized clinical trial, Kutner and colleagues (2008)23 showed that MT had immediate beneficial effects on pain and mood among patients with advanced cancer. In their study, both the massage and the simple touch groups had significant improvements in pain and quality of life over time without any increases in analgesic medication use.23 In the present study, pain in the chest, back and shoulders was similar in both the control and the MT groups at the start of the study and decreased in a similar manner over the 3 days. This decrease in pain during the postoperative period has been previously described in patients following heart surgery.24 Reduction in pain during the recovery period following surgery may in part be explained by the generation of humoral mediators (e.g., endorphins) that contribute to an increasing sense of wellbeing.1 In a recent study, Albert and colleagues (2009)25 found no additional reduction in pain in patients who were subjected to MT both before and after heart surgery. This study suggests that MT is most effective in reducing pain when it is applied post-surgery. Therefore, our study allows us to conclude that the beneficial effects of MT on sleep are unlikely to be mediated by pain reduction.
The present study had both strengths and limitations. The design allowed for the inclusion of two groups that were similar in preoperative, intraoperative, and postoperative characteristics. Patients randomized to the control group had a significantly higher BMI than those in the MT group. However, both groups had a mean BMI in the overweight range (28.5 kg/m2 and 25.9 kg/m2 in the control and MT groups, respectively, Table 1). Therefore, an imbalance in participant BMI had no influence on the results of the present study. The primary limitation of the study is that we employed self-reported, subjective methods and did not perform polysomnography to evaluate the effects of MT. Patients with cardiac disease present a high prevalence of sleep disordered breathing, that was not objectively investigated in this study.26 However, the study evaluated complaints of fatigue, pain, and sleep, which are intrinsically subjective. Moreover, our observation that the pain complaints were similar in both the control and the MT groups suggests that our evaluation was consistent and valid.
CONCLUSIONIn conclusion, our data suggest that MT is effective at improving the quality of sleep and decreasing fatigue in patients during the recovery period following CABG surgery.
Trial Registration: NCT01095419