The objective of this systematic review is to provide efficacy and safety data in the application of Intra-Abdominal Hyperthermia Chemotherapy (HIPEC) and Cytoreductive Surgery (CRS) in patients with Peritoneal Pseudomyxoma (PMP) of origin in the cecal appendix. The databases Medline and Central Cochrane were consulted. Patients with PMP of origin in the cecal appendix, classified as low grade, high or indeterminate, submitted to HIPEC and CRS. The results were meta-analyzed using the Comprehensive Metanalysis software. Twenty-six studies were selected to support this review. For low-grade PMP outcome, 60-month risk of mortality, Disease-Free Survival (DFS), and adverse events was 28.8% (95% CI 25.9 to 32), 43% (95% CI 36.4 and 49.8), and 46.7% (95% CI 40.7 to 52.8); for high-grade PMP, 60-month risk of mortality, Disease-Free Survival (DFS) and adverse events was 55.9% (95% CI 51.9 to 59.6), 20.1% (95% CI 15.5 to 25.7) and 30% (95% CI 25.2 to 35.3); PMP indeterminate degree, 60-month risk of mortality, Disease-Free Survival (DFS) and adverse events was 32.6% (95% CI 30.5 to 34.7), 61.8% (95% CI 58.8 to 64.7) and 32.9% (95% CI 30.5 to 35.4). The authors conclude that the HIPEC technique and cytoreductive surgery can be applied to selected cases of patients with PMP of peritoneal origin with satisfactory results.
Peritoneal Pseudomyxoma (PMP) was first described by Rokitansky in 1842;1 Werth, in 1884,2 introduced the term peritoneal pseudomyxoma, describing ovarian mucinous carcinoma and presence of gelatinous ascites "("jelly belly""). In 1901, Frankel described the first case of peritoneal pseuxomyxomatous syndrome resulting from cystic rupture in cecal appendix.
This disease is a rare type of cancer that involves the peritoneal surface, whose most common origin is the cecal appendix, but also occurs in other places such as stomach, colon, meso or ovarian. It is characterized by the large production of mucin, with consequent mucinous ascites.
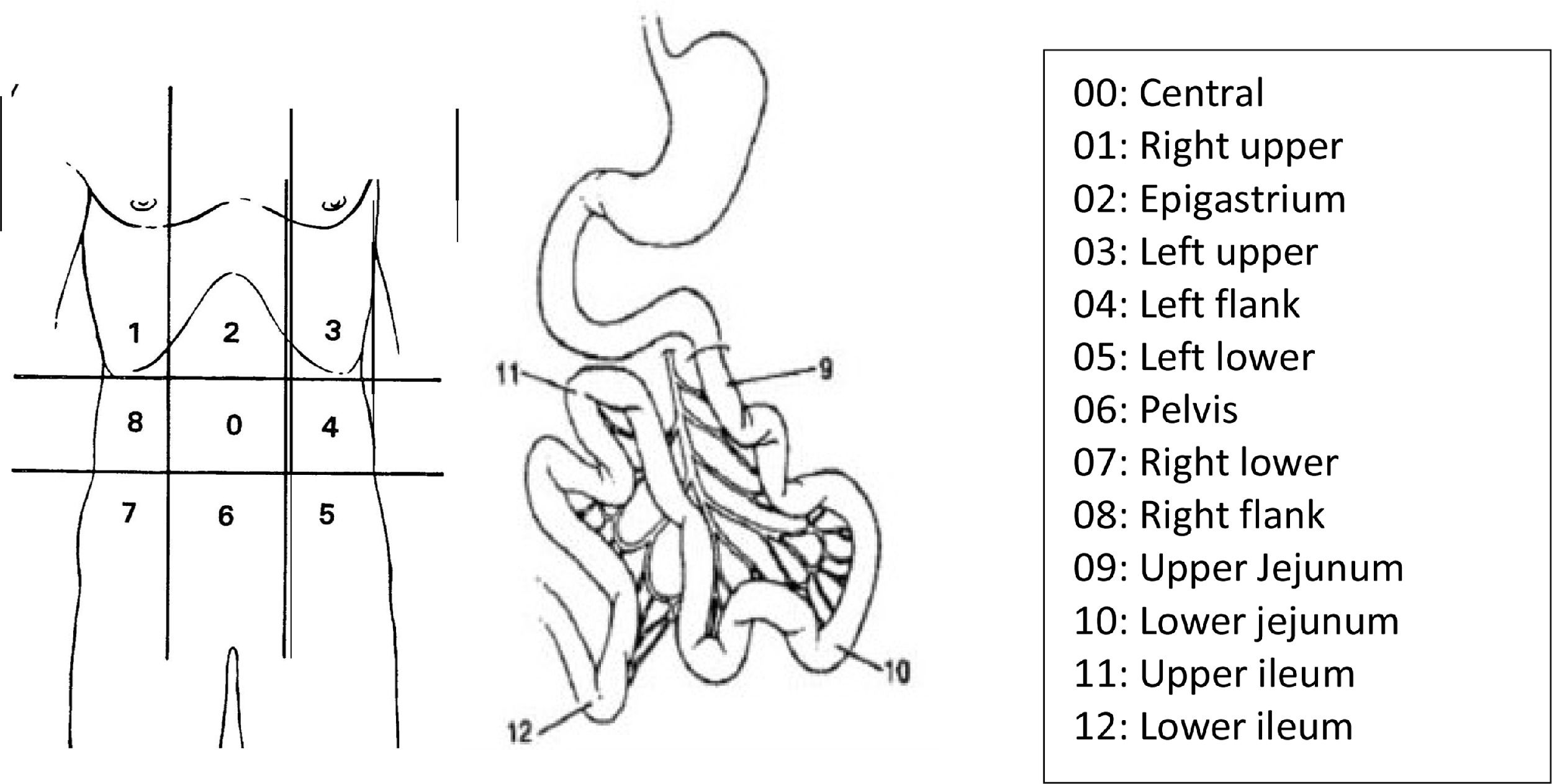
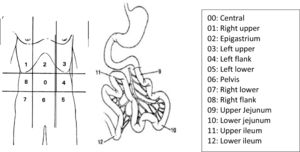
In 1995, Sugarbaker3 quantified the dispersion of abdominal disease through numerical values correlated to quadrants of the abdomen, determining the Peritoneal Carcinomatosis Index (PCI), according to the classification below (Fig. 1).
The surgical treatment applied PMP is performed through Peritoneal Cytoreductive surgery (CCP) that can be surgically classified5 in:
- •
CC-0 - No residual tumor (= R0 resection) (en bloc resection);
- •
CC-1 ‒ < 0.25 cm residual tumor tissue (complete cytoreduction);
- •
CC-2 ‒ 0.25–2.5 cm residual tumor tissue (incomplete cytoreduction with moderate residual tumor proportion);
- •
CC-3 ‒ > 2.5 cm residual tumor tissue (incomplete cytoreduction with high residual tumor proportion).
The Consensus6 was achieved on the pathologic classification of PMP, defined as the intraperitoneal accumulation of mucus due to mucinous neoplasia characterized by the redistribution phenomenon and classified:
- 1
Mucin without epithelial cells.
- 2
PMP with Low-grade. Low-grade mucinous peritoneal carcinoma or Dissemination Peritoneal Adenomatosis (DPAM).
- 3
PMP with High-grade. High-grade mucinous carcinoma peritonei or Peritoneal Mucinous Carcinomatosis (PMCA).
- 4
PMP with signet ring cells. High-grade mucinous carcinoma peritonei with signet ring cells OR Peritoneal Mucinous Carcinomatosis with Signet ring cells (PMCA-S).
Intraoperative adjuvant treatment can be applied through Peritoneal Hyperthermic Chemotherapy (HIPEC). The technique described by Spratt et al.7 Mitomycin, Oxaliplatin, or Cisplatin chemotherapy are currently used intraoperatively, which have been heated for 42 degrees.
ObjectiveTo evaluate the efficacy and safety in the application of intra-abdominal hyperthermic chemotherapy and cytoreductive surgery for patients with pseudomyxoma peritonei from the cecal appendix.
MethodsThe protocol of this study has been registered in PROSPERO (CRD42021252820). This systematic review will be prepared according to recommendations contained in PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).8
The eligibility criteria of the studies are:
- 1
Adult patient with PMP from cecal appendix;
- 2
Treatment – CRS and HIPEC;
- 3
Outcomes ‒ Mortality, disease-free survival, and adverse events of any cause, degree ≥ 3;9
- 4
Follow-up time up to 60-months;
- 5
Randomized controlled trials, comparative non-randomized studies and case series;
- 6
No period or language limit;
- 7
Full text available for access.
The search for evidence will be conducted on the following virtual scientific information databases, using the search strategies:
Medline/PubMed: ([Pseudomyxoma peritonei OR syndrome of pseudomyxoma peritoneal OR gelatinous ascites] AND [hyperthermic intraperitoneal chemotherapy]);
Central Cochrane: (Pseudomyxoma peritonei AND hyperthermic intraperitoneal chemotherapy).
The information obtained from the characteristics of the studies were: 'author's name and year of the study, study design, number of patients, population, methods of intervention and comparison, absolute number of outcomes, and follow-up.
The measurement used to express benefit and damage varied according to outcomes expressed by means of continuous variables (mean and standard deviation) or expressed by categorical variables (absolute number of events). In continuous measurement, the results are of difference in means and standard deviation, and in categorical measures, the results are of absolute risks, differences in risks, and number needed to treat or to produce damage, considering the number of patients. The confidence level used will be 95%. When in the presence of common outcomes among the included studies, the results will be expressed through meta-analysis.
Bias assessment and quality of evidenceCase series studies or before and after will have their risk of bias analyzed according to the Joanna Briggs Institute Critical instrument.10 Cohort and case-control studies will be evaluated with the Robins – I instrument11 tool, while randomized clinical trials will have their risk of bias analyzed using the RoB 2 instrument.12
The results of comparative observational clinical trials will be aggregated and meta-analyzed using Revman 5.413 software, while non-comparative studies will be meta-analyzed using the Comprehensive Metanalysis software.
Furthermore, the quality of evidence will be graded as high, moderate, low, or very low using the Grade instrument14 and considering the risk of bias, the presence of inconsistency, inaccuracy, or indirect evidence in the meta-analysis of the outcomes, and the presence of publication bias.
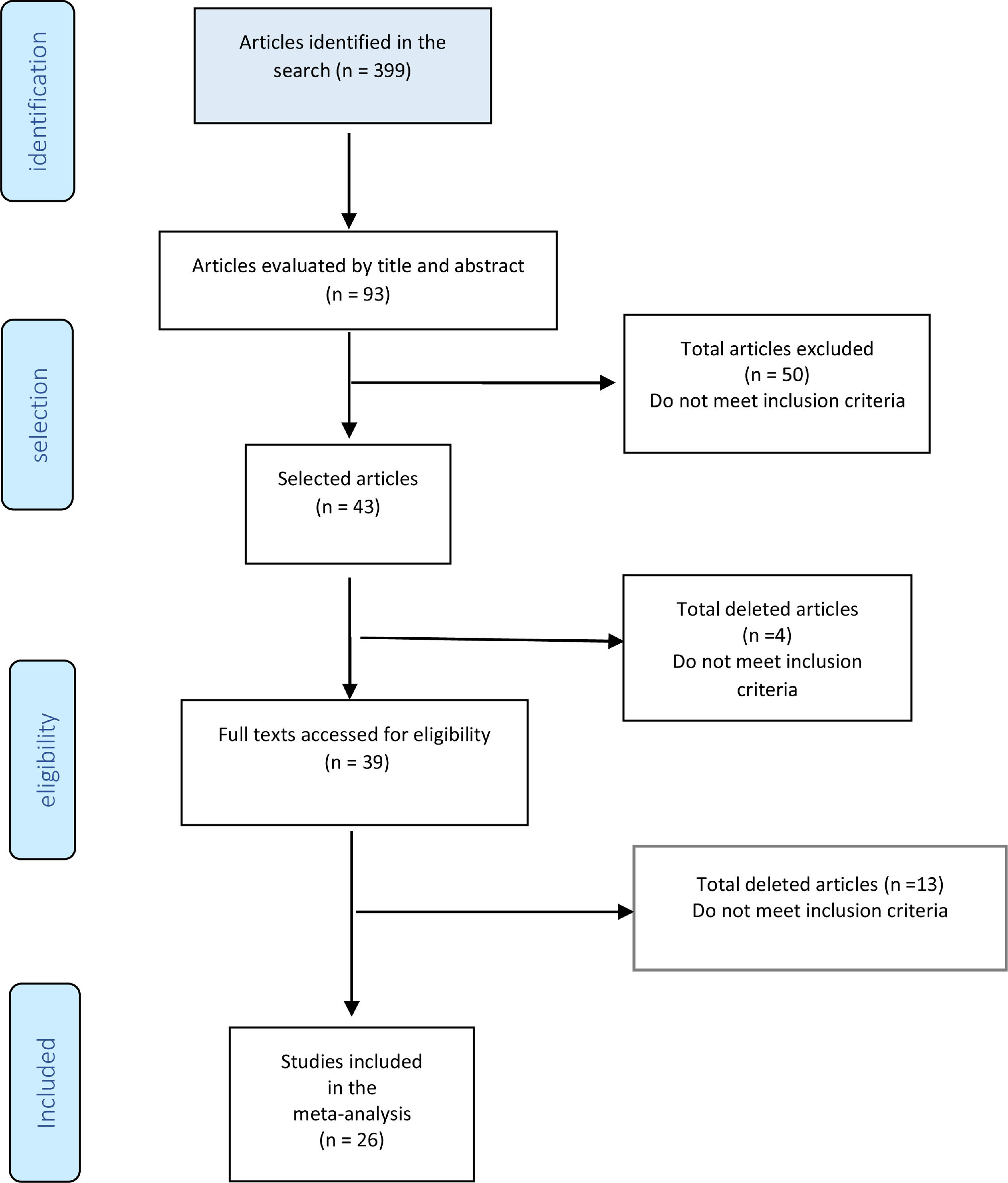
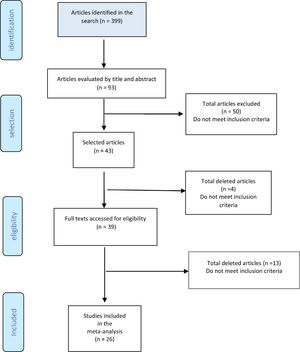
ResultsFig. 10 shows the study diagram. As of January 2021, the search strategy identified 399 studies with titles and abstracts, and screening identified 94 potentially eligible citations. The full-test screening of 43 citations identified 26 studies15-40 as potentially relevant publications, all studies were case series. The reasons for exclusion and the list of excluded studies are available in the references, ANNEXES (Fig. 2 and Table 1). The result was extracted in absolute numbers and meta-analyzed in absolute risk, without comparison.
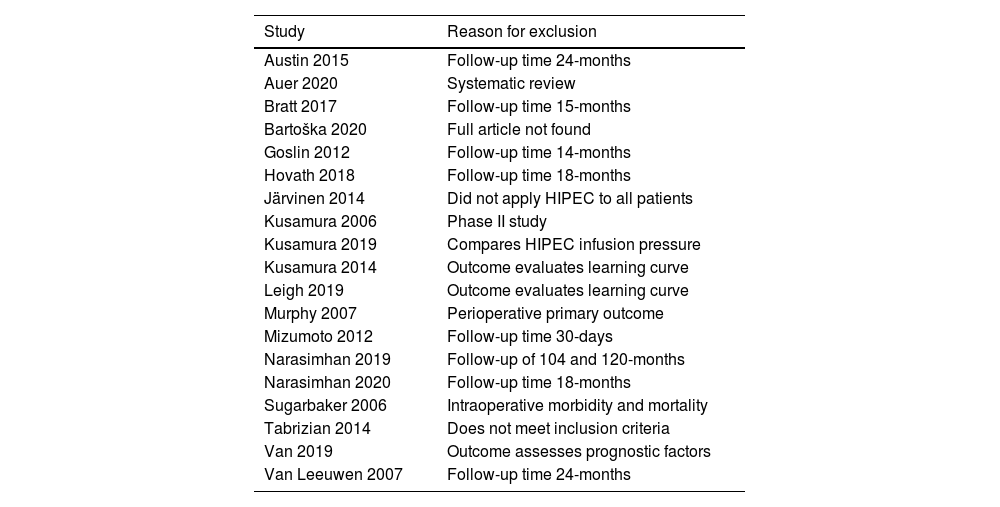
Excluded articles and reason for exclusion.
| Study | Reason for exclusion |
|---|---|
| Austin 2015 | Follow-up time 24-months |
| Auer 2020 | Systematic review |
| Bratt 2017 | Follow-up time 15-months |
| Bartoška 2020 | Full article not found |
| Goslin 2012 | Follow-up time 14-months |
| Hovath 2018 | Follow-up time 18-months |
| Järvinen 2014 | Did not apply HIPEC to all patients |
| Kusamura 2006 | Phase II study |
| Kusamura 2019 | Compares HIPEC infusion pressure |
| Kusamura 2014 | Outcome evaluates learning curve |
| Leigh 2019 | Outcome evaluates learning curve |
| Murphy 2007 | Perioperative primary outcome |
| Mizumoto 2012 | Follow-up time 30-days |
| Narasimhan 2019 | Follow-up of 104 and 120-months |
| Narasimhan 2020 | Follow-up time 18-months |
| Sugarbaker 2006 | Intraoperative morbidity and mortality |
| Tabrizian 2014 | Does not meet inclusion criteria |
| Van 2019 | Outcome assesses prognostic factors |
| Van Leeuwen 2007 | Follow-up time 24-months |
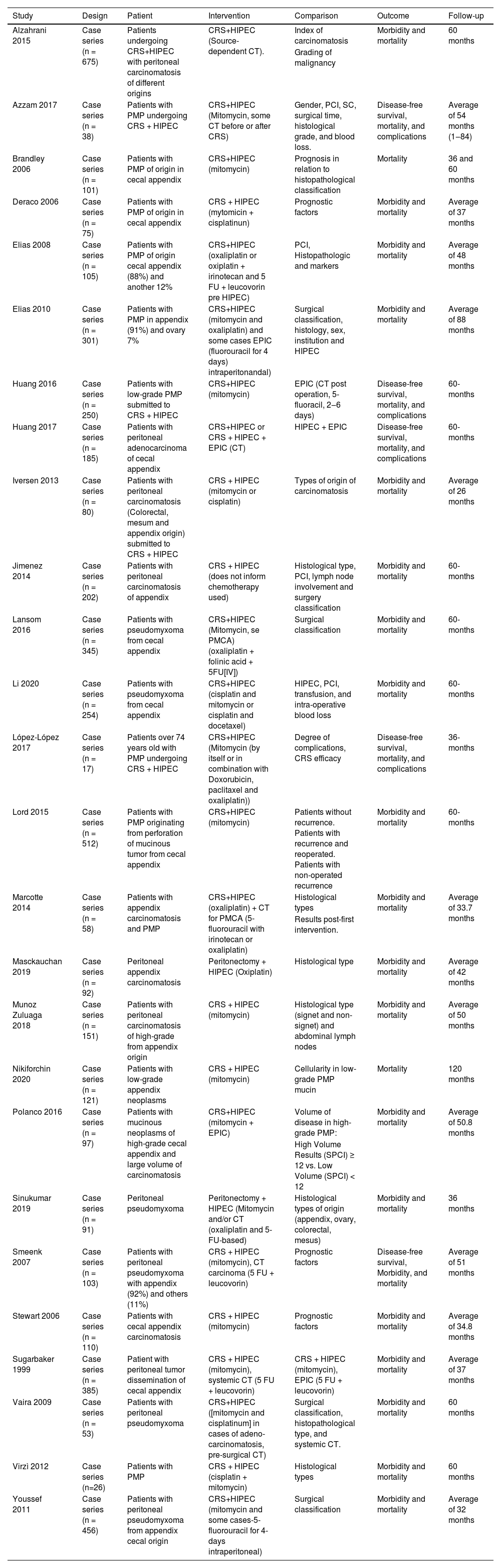
The present study included population was a total of 3.274 patients with PMP from the cecal appendix, submitted to HIPEC and CCR treatment, followed for analysis of outcomes death, disease-free survival, and adverse effects in a mean follow-up of 36 and 60 months. Characteristics of the selected studies are described in Table 2, in annexes.
Description of the included studies RCC associated with HIPEC in peritoneal pseudomyxoma originating from the cecal appendix.
| Study | Design | Patient | Intervention | Comparison | Outcome | Follow-up |
|---|---|---|---|---|---|---|
| Alzahrani 2015 | Case series (n = 675) | Patients undergoing CRS+HIPEC with peritoneal carcinomatosis of different origins | CRS+HIPEC (Source-dependent CT). | Index of carcinomatosis | Morbidity and mortality | 60 months |
| Grading of malignancy | ||||||
| Azzam 2017 | Case series (n = 38) | Patients with PMP undergoing CRS + HIPEC | CRS+HIPEC (Mitomycin, some CT before or after CRS) | Gender, PCI, SC, surgical time, histological grade, and blood loss. | Disease-free survival, mortality, and complications | Average of 54 months (1‒84) |
| Brandley 2006 | Case series (n = 101) | Patients with PMP of origin in cecal appendix | CRS+HIPEC (mitomycin) | Prognosis in relation to histopathological classification | Mortality | 36 and 60 months |
| Deraco 2006 | Case series (n = 75) | Patients with PMP of origin in cecal appendix | CRS + HIPEC (mytomicin + cisplatinun) | Prognostic factors | Morbidity and mortality | Average of 37 months |
| Elias 2008 | Case series (n = 105) | Patients with PMP of origin cecal appendix (88%) and another 12% | CRS+HIPEC (oxaliplatin or oxiplatin + irinotecan and 5 FU + leucovorin pre HIPEC) | PCI, Histopathologic and markers | Morbidity and mortality | Average of 48 months |
| Elias 2010 | Case series (n = 301) | Patients with PMP in appendix (91%) and ovary 7% | CRS+HIPEC (mitomycin and oxaliplatin) and some cases EPIC (fluorouracil for 4 days) intraperitonandal) | Surgical classification, histology, sex, institution and HIPEC | Morbidity and mortality | Average of 88 months |
| Huang 2016 | Case series (n = 250) | Patients with low-grade PMP submitted to CRS + HIPEC | CRS+HIPEC (mitomycin) | EPIC (CT post operation, 5-fluoracil, 2‒6 days) | Disease-free survival, mortality, and complications | 60-months |
| Huang 2017 | Case series (n = 185) | Patients with peritoneal adenocarcinoma of cecal appendix | CRS+HIPEC or CRS + HIPEC + EPIC (CT) | HIPEC + EPIC | Disease-free survival, mortality, and complications | 60-months |
| Iversen 2013 | Case series (n = 80) | Patients with peritoneal carcinomatosis (Colorectal, mesum and appendix origin) submitted to CRS + HIPEC | CRS + HIPEC (mitomycin or cisplatin) | Types of origin of carcinomatosis | Morbidity and mortality | Average of 26 months |
| Jimenez 2014 | Case series (n = 202) | Patients with peritoneal carcinomatosis of appendix | CRS + HIPEC (does not inform chemotherapy used) | Histological type, PCI, lymph node involvement and surgery classification | Morbidity and mortality | 60-months |
| Lansom 2016 | Case series (n = 345) | Patients with pseudomyxoma from cecal appendix | CRS+HIPEC (Mitomycin, se PMCA) (oxaliplatin + folinic acid + 5FU[IV]) | Surgical classification | Morbidity and mortality | 60-months |
| Li 2020 | Case series (n = 254) | Patients with pseudomyxoma from cecal appendix | CRS+HIPEC (cisplatin and mitomycin or cisplatin and docetaxel) | HIPEC, PCI, transfusion, and intra-operative blood loss | Morbidity and mortality | 60-months |
| López-López 2017 | Case series (n = 17) | Patients over 74 years old with PMP undergoing CRS + HIPEC | CRS+HIPEC (Mitomycin (by itself or in combination with Doxorubicin, paclitaxel and oxaliplatin)) | Degree of complications, CRS efficacy | Disease-free survival, mortality, and complications | 36-months |
| Lord 2015 | Case series (n = 512) | Patients with PMP originating from perforation of mucinous tumor from cecal appendix | CRS+HIPEC (mitomycin) | Patients without recurrence. Patients with recurrence and reoperated. Patients with non-operated recurrence | Morbidity and mortality | 60-months |
| Marcotte 2014 | Case series (n = 58) | Patients with appendix carcinomatosis and PMP | CRS+HIPEC (oxaliplatin) + CT for PMCA (5-fluorouracil with irinotecan or oxaliplatin) | Histological types | Morbidity and mortality | Average of 33.7 months |
| Results post-first intervention. | ||||||
| Masckauchan 2019 | Case series (n = 92) | Peritoneal appendix carcinomatosis | Peritonectomy + HIPEC (Oxiplatin) | Histological type | Morbidity and mortality | Average of 42 months |
| Munoz Zuluaga 2018 | Case series (n = 151) | Patients with peritoneal carcinomatosis of high-grade from appendix origin | CRS + HIPEC (mitomycin) | Histological type (signet and non-signet) and abdominal lymph nodes | Morbidity and mortality | Average of 50 months |
| Nikiforchin 2020 | Case series (n = 121) | Patients with low-grade appendix neoplasms | CRS + HIPEC (mitomycin) | Cellularity in low-grade PMP mucin | Mortality | 120 months |
| Polanco 2016 | Case series (n = 97) | Patients with mucinous neoplasms of high-grade cecal appendix and large volume of carcinomatosis | CRS+HIPEC (mitomycin + EPIC) | Volume of disease in high-grade PMP: | Morbidity and mortality | Average of 50.8 months |
| High Volume Results (SPCI) ≥ 12 vs. Low Volume (SPCI) < 12 | ||||||
| Sinukumar 2019 | Case series (n = 91) | Peritoneal pseudomyxoma | Peritonectomy + HIPEC (Mitomycin and/or CT (oxaliplatin and 5-FU-based) | Histological types of origin (appendix, ovary, colorectal, mesus) | Morbidity and mortality | 36 months |
| Smeenk 2007 | Case series (n = 103) | Patients with peritoneal pseudomyxoma with appendix (92%) and others (11%) | CRS + HIPEC (mitomycin), CT carcinoma (5 FU + leucovorin) | Prognostic factors | Disease-free survival, Morbidity, and mortality | Average of 51 months |
| Stewart 2006 | Case series (n = 110) | Patients with cecal appendix carcinomatosis | CRS + HIPEC (mitomycin) | Prognostic factors | Morbidity and mortality | Average of 34.8 months |
| Sugarbaker 1999 | Case series (n = 385) | Patient with peritoneal tumor dissemination of cecal appendix | CRS + HIPEC (mitomycin), systemic CT (5 FU + leucovorin) | CRS + HIPEC (mitomycin), EPIC (5 FU + leucovorin) | Morbidity and mortality | Average of 37 months |
| Vaira 2009 | Case series (n = 53) | Patients with peritoneal pseudomyxoma | CRS+HIPEC ([mitomycin and cisplatinum] in cases of adeno-carcinomatosis, pre-surgical CT) | Surgical classification, histopathological type, and systemic CT. | Morbidity and mortality | 60 months |
| Virzì 2012 | Case series (n=26) | Patients with PMP | CRS + HIPEC (cisplatin + mitomycin) | Histological types | Morbidity and mortality | 60 months |
| Youssef 2011 | Case series (n = 456) | Patients with peritoneal pseudomyxoma from appendix cecal origin | CRS+HIPEC (mitomycin and some cases-5-fluorouracil for 4-days intraperitoneal) | Surgical classification | Morbidity and mortality | Average of 32 months |
CRS, Cytoreductive Surgery; HIPEC, Intraperitoneal Chemotherapy; PCI, Peritoneal Carcinomatosis Index; CT, Chemotherapy; PMP, Peritoneal Pseudomyxoma; SC, Surgical Classification; EPIC, Early Postoperative Intraperitoneal Chemotherapy; PMCA, Peritoneal Mucinous Carcinomatosis; SPCI, Simplified Peritoneal Cancer.
NiKiforchin et al.,32 evaluated as prognostic factor cellularity in ascytic fluid in low-grade PMP: defined as acellular or cellular ascitic liquid, in the extraction of the results, both outcomes were added. Sugarbaker and Chang37 evaluated complete and incomplete cytoreductive surgery, the results used for meta-analysis were only from complete surgery. Munhoz-Zuluaga et al.,31 evaluated High-Grade Peritoneal Mucinous Carcinoma (HGMCP) and High-Grade Peritoneal Mucinous Carcinoma with Synet cells (HGMCP-S). During the study data extraction, both results were added to the outcomes in HGMCP and HGMCP-S. Polanco et al.,33 evaluated High-Volume (HV) disease as defined as SPCI C < 12, while SPCI > 12 was considered Low-Volume (LV) disease, and the results used were the sum of both for high-grade PMP outcomes. Huang Y et al.,22 evaluated patients with PMP without histopathological classification, submitted to HIPEC or HIPEC associated with Perioperative Chemotherapy (EPIC) (2‒6 days), data were collected only from patients submitted to HPIEC.
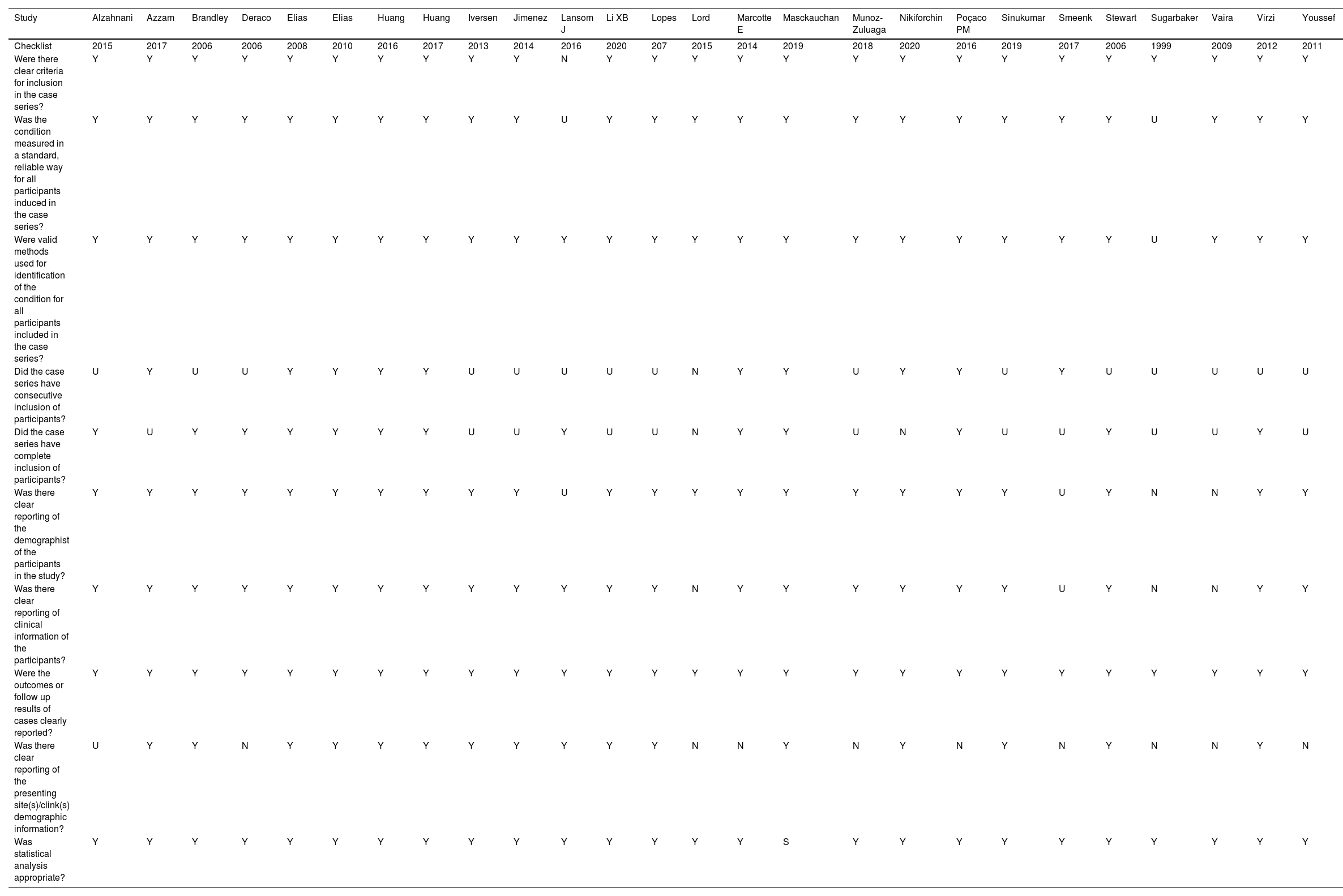
The judgments for the risk of bias of the 26 studies15-40 were analyzed by the Joanna Briggs Institute Critical10 instrument: 80% presented low risk, 16% moderate risk, and 4% high risk. Results were summarised in a risk of bias graph (Table 3).
Description of the biases of the included studies, for peritoneal pseudomyxoma of cecal appendix origin. Criteria of Joanna Briggs Institute Critical.
| Study | Alzahnani | Azzam | Brandley | Deraco | Elias | Elias | Huang | Huang | Iversen | Jimenez | Lansom J | Li XB | Lopes | Lord | Marcotte E | Masckauchan | Munoz-Zuluaga | Nikiforchin | Poçaco PM | Sinukumar | Smeenk | Stewart | Sugarbaker | Vaira | Virzi | Youssef |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Checklist | 2015 | 2017 | 2006 | 2006 | 2008 | 2010 | 2016 | 2017 | 2013 | 2014 | 2016 | 2020 | 207 | 2015 | 2014 | 2019 | 2018 | 2020 | 2016 | 2019 | 2017 | 2006 | 1999 | 2009 | 2012 | 2011 |
| Were there clear criteria for inclusion in the case series? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the condition measured in a standard, reliable way for all participants induced in the case series? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Were valid methods used for identification of the condition for all participants included in the case series? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Did the case series have consecutive inclusion of participants? | U | Y | U | U | Y | Y | Y | Y | U | U | U | U | U | N | Y | Y | U | Y | Y | U | Y | U | U | U | U | U |
| Did the case series have complete inclusion of participants? | Y | U | Y | Y | Y | Y | Y | Y | U | U | Y | U | U | N | Y | Y | U | N | Y | U | U | Y | U | U | Y | U |
| Was there clear reporting of the demographist of the participants in the study? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | N | N | Y | Y |
| Was there clear reporting of clinical information of the participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | U | Y | N | N | Y | Y |
| Were the outcomes or follow up results of cases clearly reported? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was there clear reporting of the presenting site(s)/clink(s) demographic information? | U | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | Y | N | Y | N | Y | N | N | Y | N |
| Was statistical analysis appropriate? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | S | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Y, Yes; N, Not; U, Unclear.
Meta-analysis of eleven clinical trials15,17,24,25,28,29,32,35-37,39 including 1043 participants found that HIPEC and CRS.
Mortality at 36-month was evaluated in three studies,32,35,36 including 242 participants. The risk of mortality was 34.4% (95% CI 28.6 and 40.7; I2 = 68.61%) (Fig. 3).
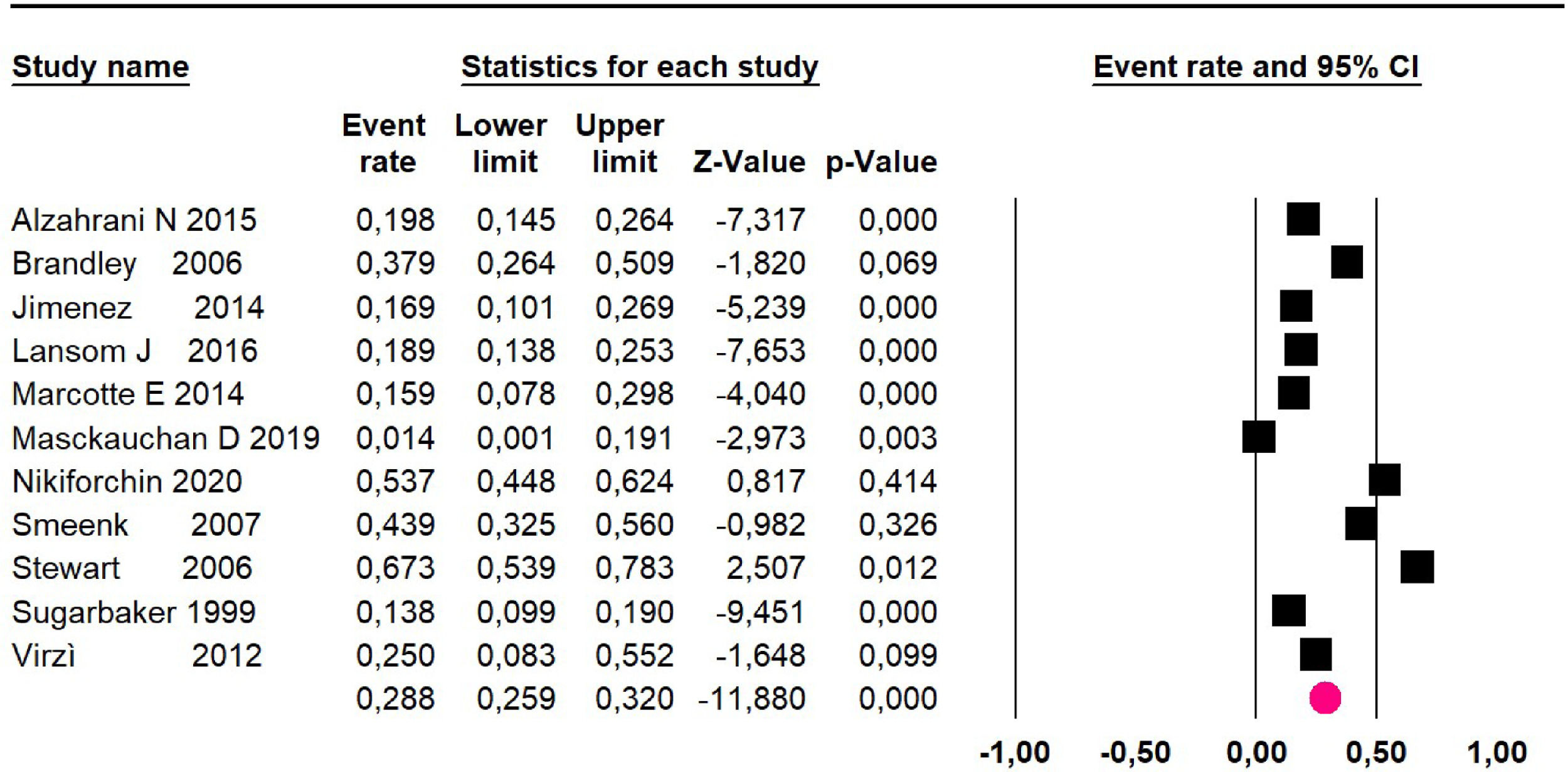
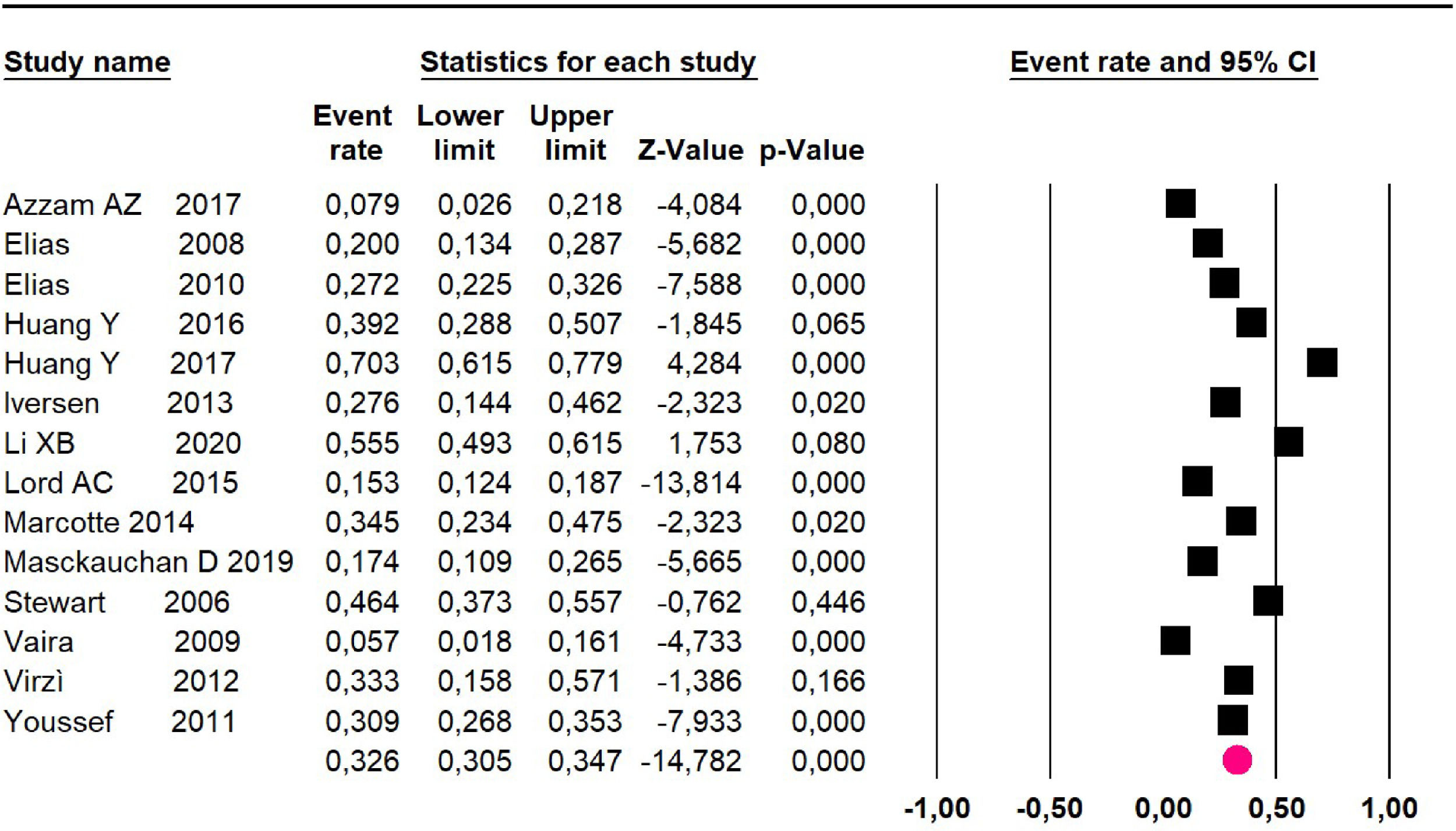
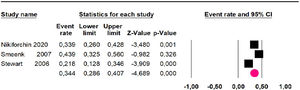
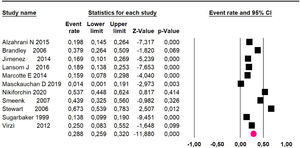
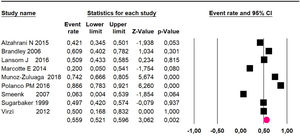
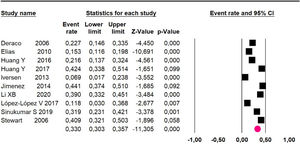
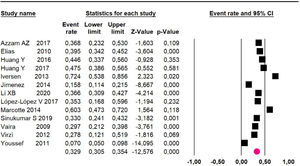
Mortality at 60-month: risk mortality was evaluated in eleven studies15,17,24,25,29,30,32,35-37,39 with 1043 patients. The risk was 28.8% (95% CI 25.9 to 32; I2 = 92.1%). Fig. 4.
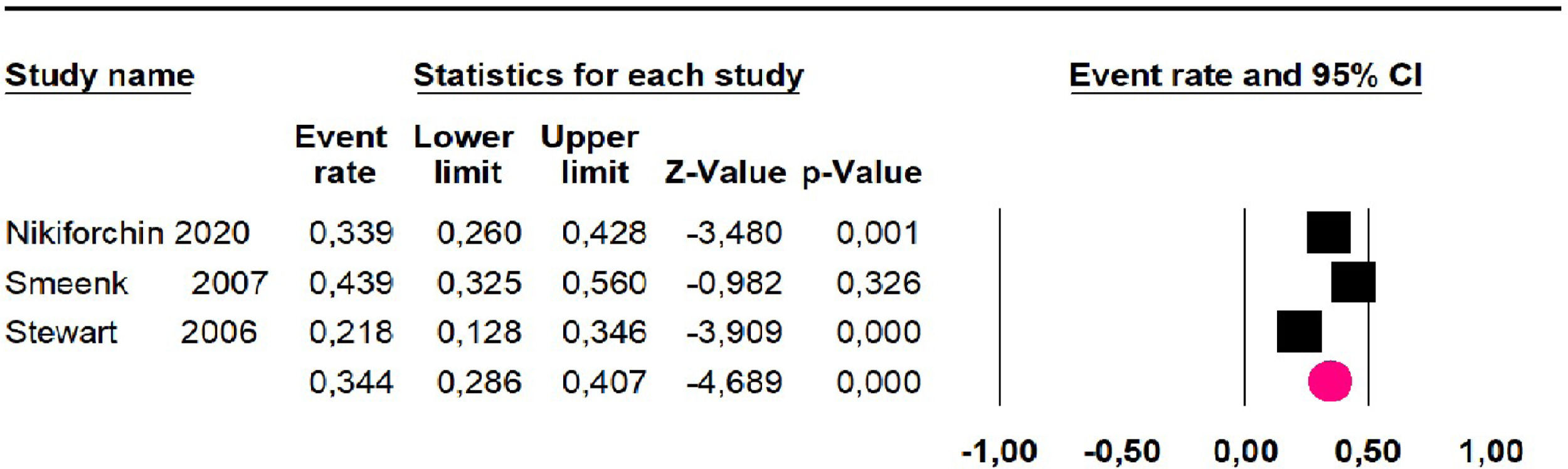
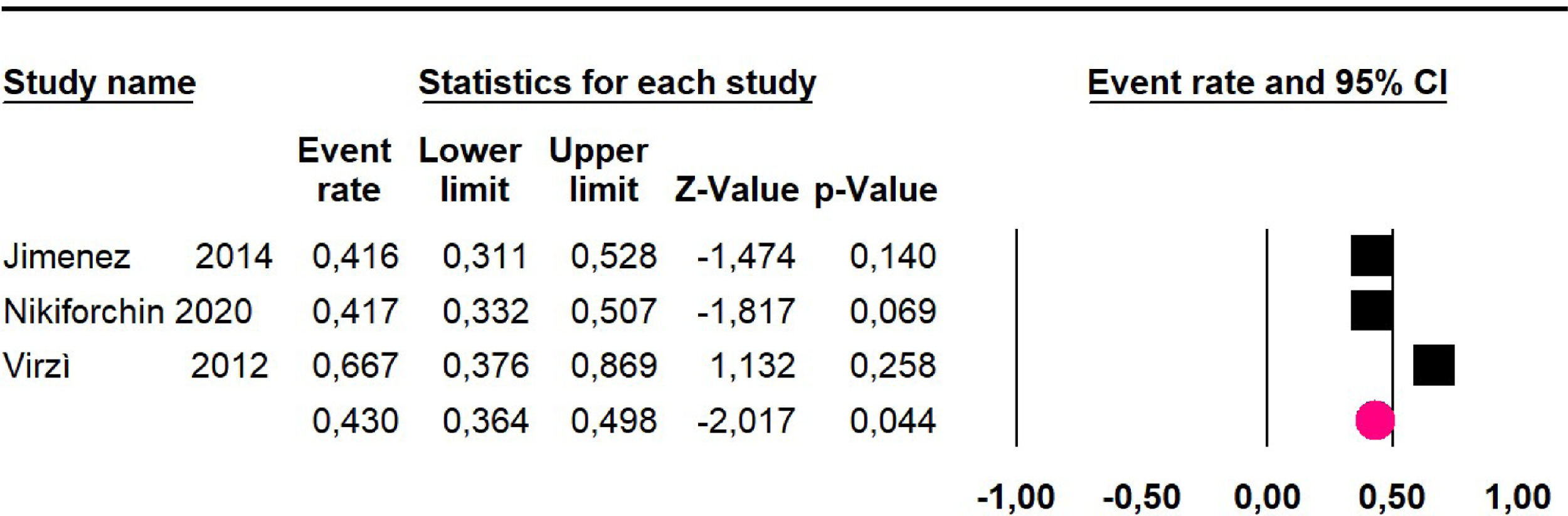
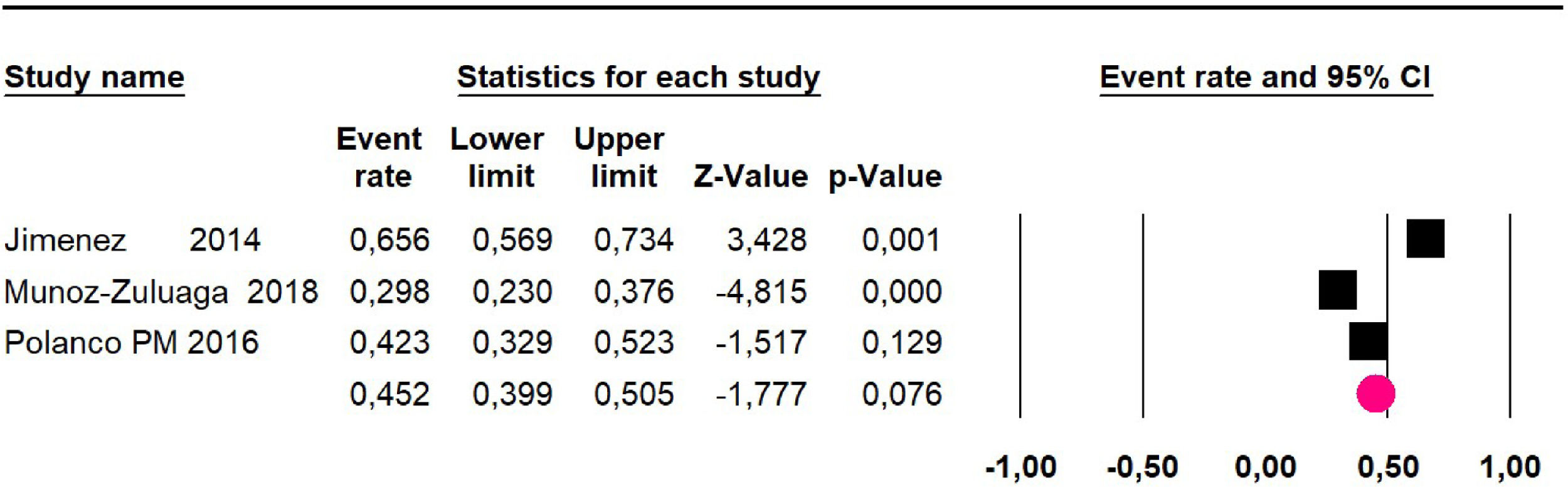
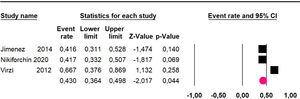
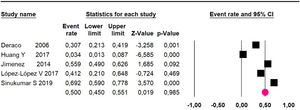
Disease-free survival: Meta-analysis of three studies,24,32,39 assessing 209 participants, the follow-up 60-month risk was 43% (95% CI 36.4 and 49.8; I2 = 25.57%) (Fig. 5).
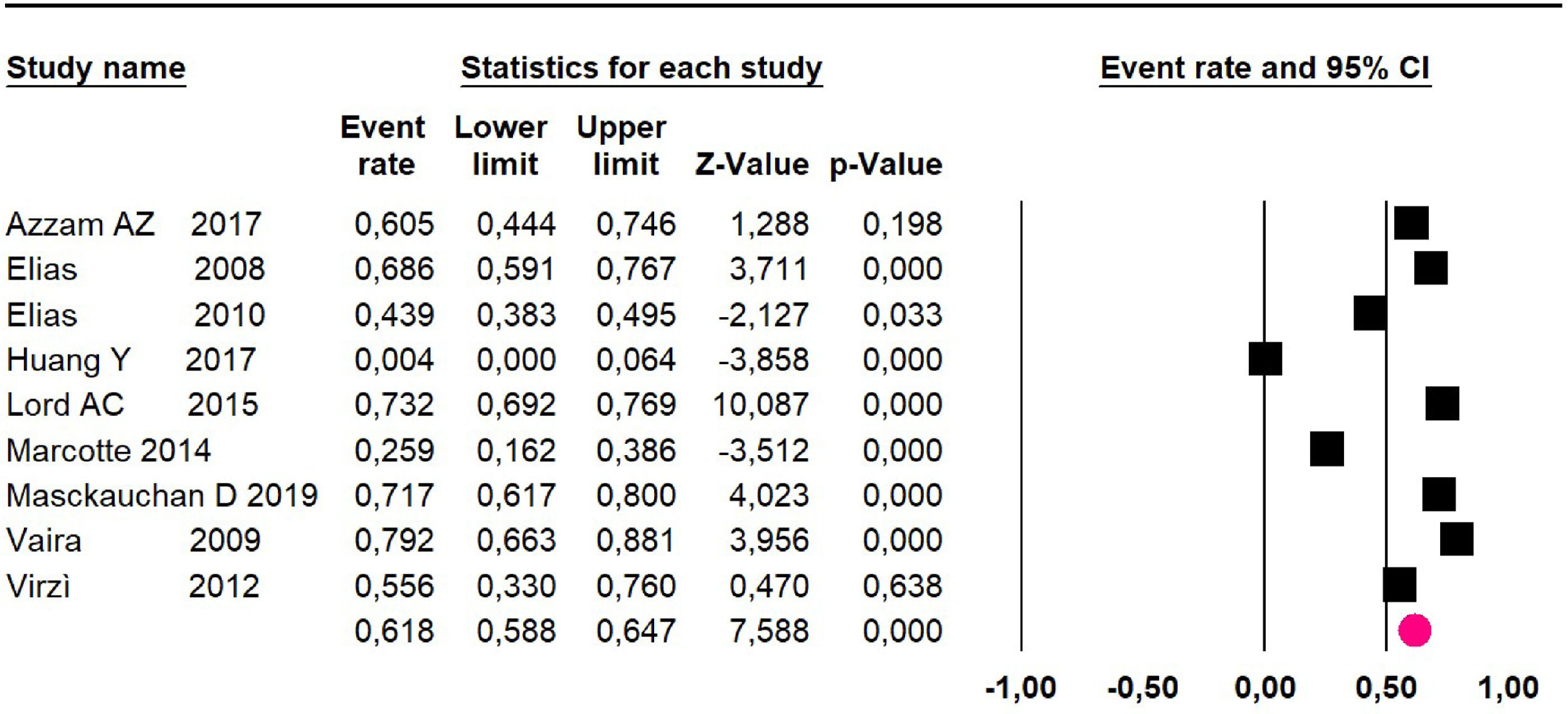
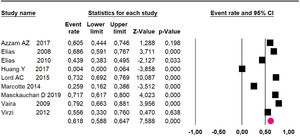
Adverse events greater than or equal to degree III: a meta-analysis of four studies24,29,32,39 with 267 patients, the 60-month risk was 46.7% (95% CI 40.7 to 52.8.3; I2 = 62.8%) (Fig. 6).
High-grade pseudomyxomaMeta-analysis of twelve studies,15,17,24,25,29,30,32,33,35,36,37,39 assessing 1073 participants, evaluated HIPEC and CRS for the outcome:
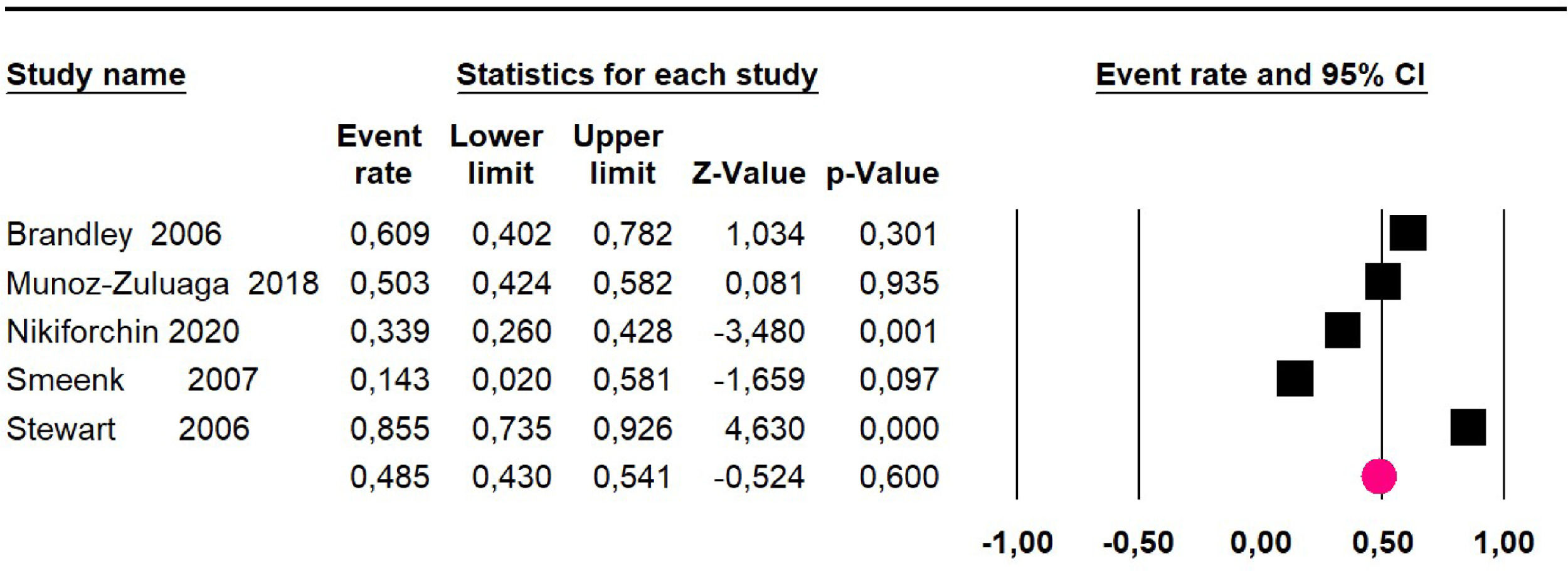
Mortality at 36-month was evaluated in five studies17,31,32,35,36 including 357 participants. The risk of mortality was 48.5% (95% CI 43% to 54.1%, I2 = 89.2%) (Fig. 7).
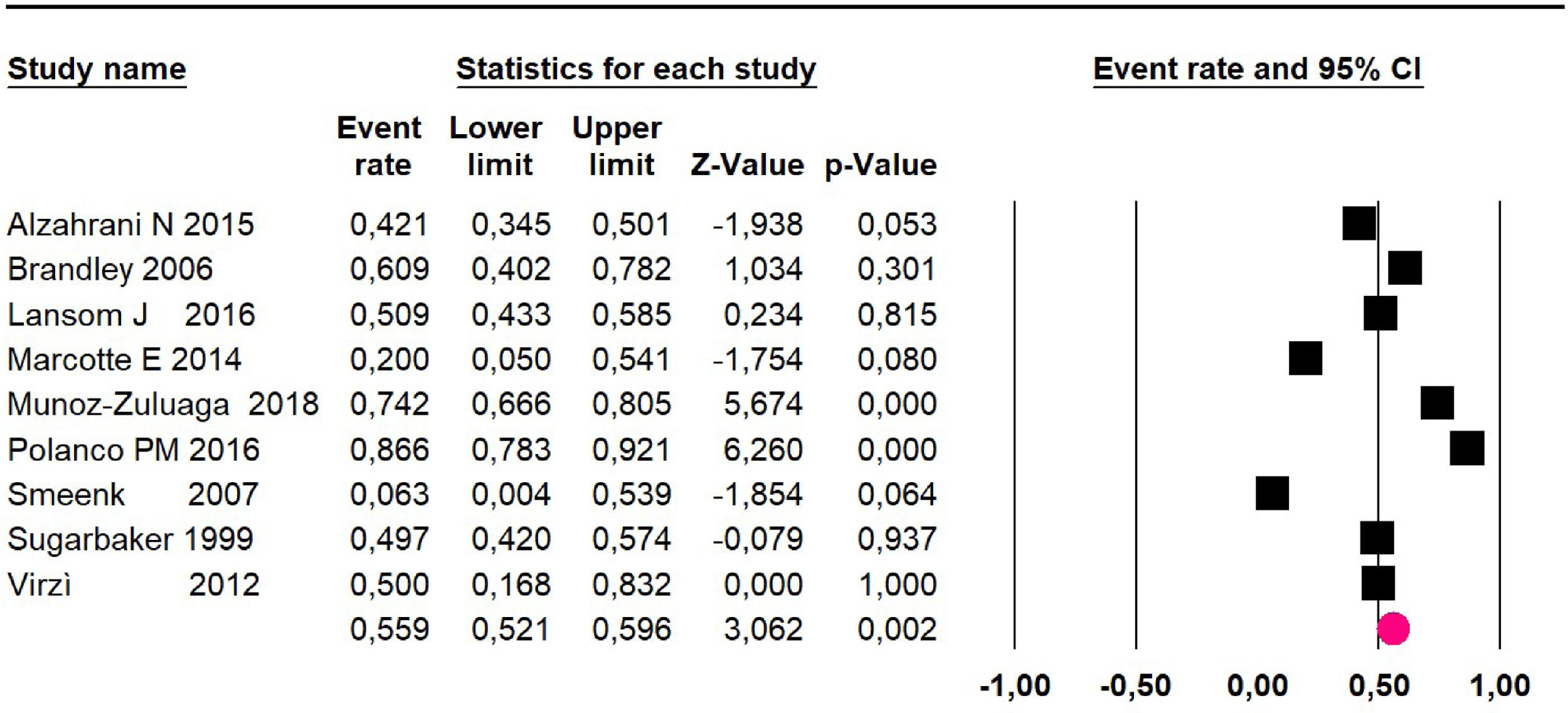
Mortality at 60-month: risk mortality was evaluated in nine studies15,17,25,29,31,33,35,37,39 including 772 patients, the risk was 55.9% (95% CI 52.1 to 59.6; I2 = 89.1%) (Fig. 8) between participants who have undergone HIPEC and CRS.
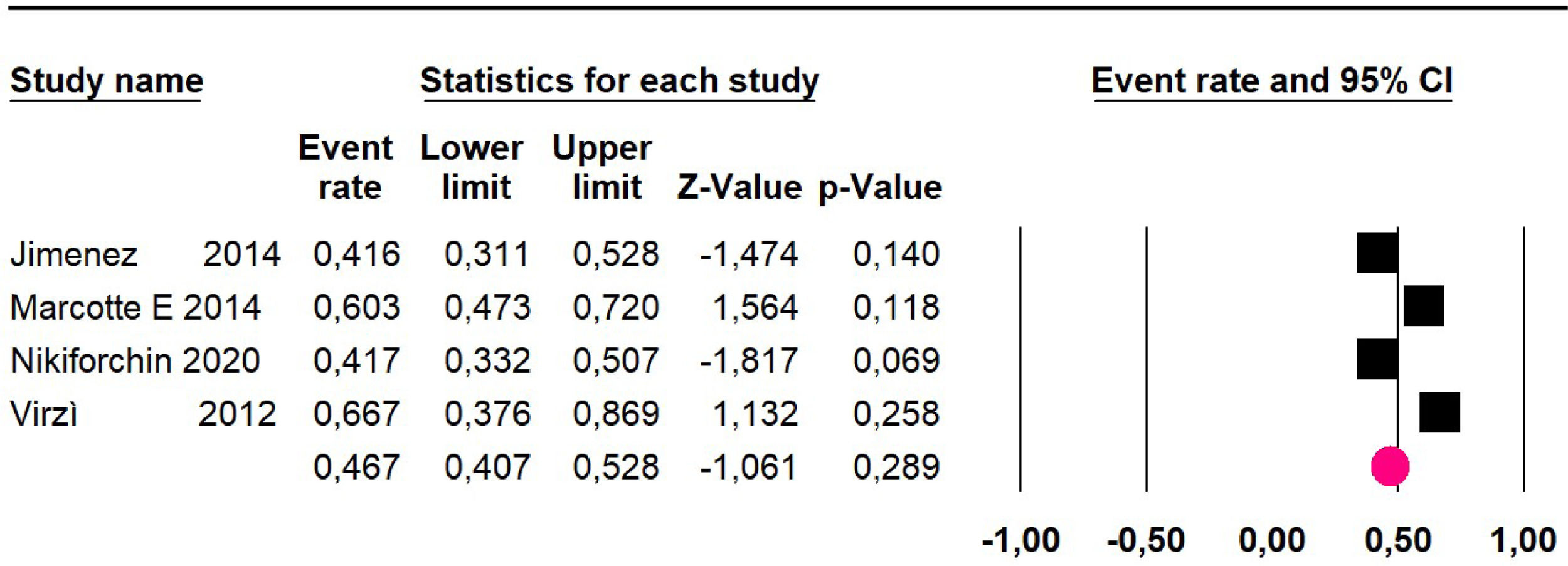
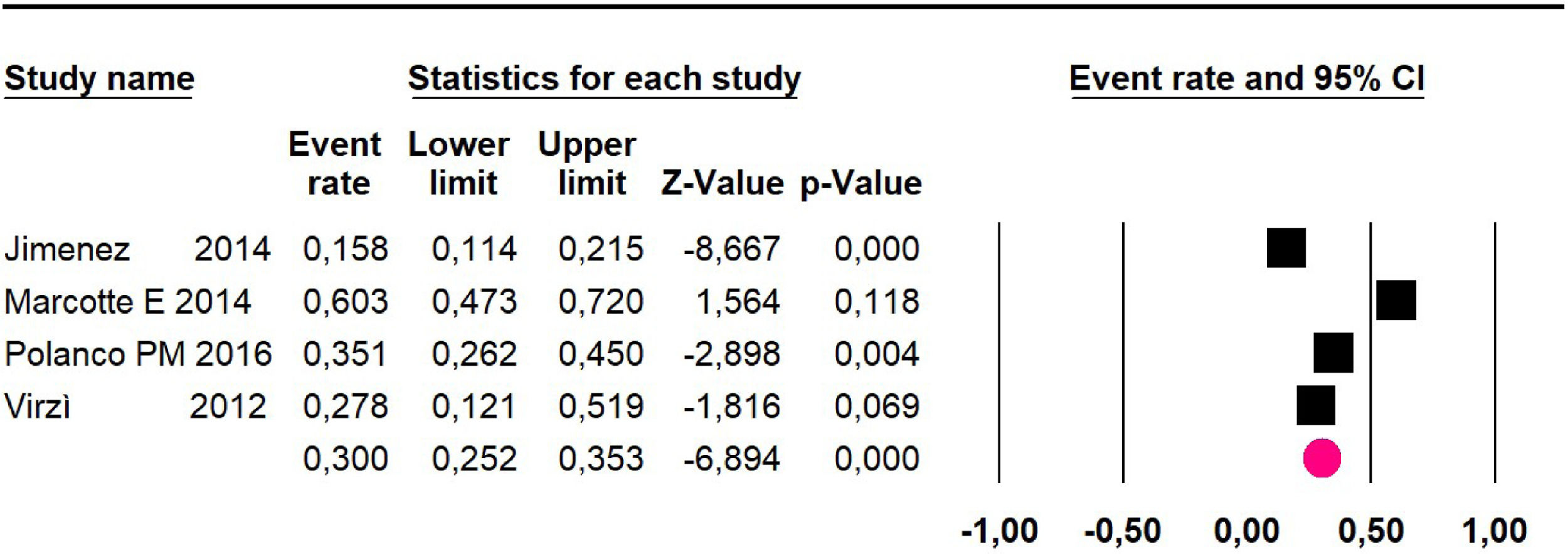
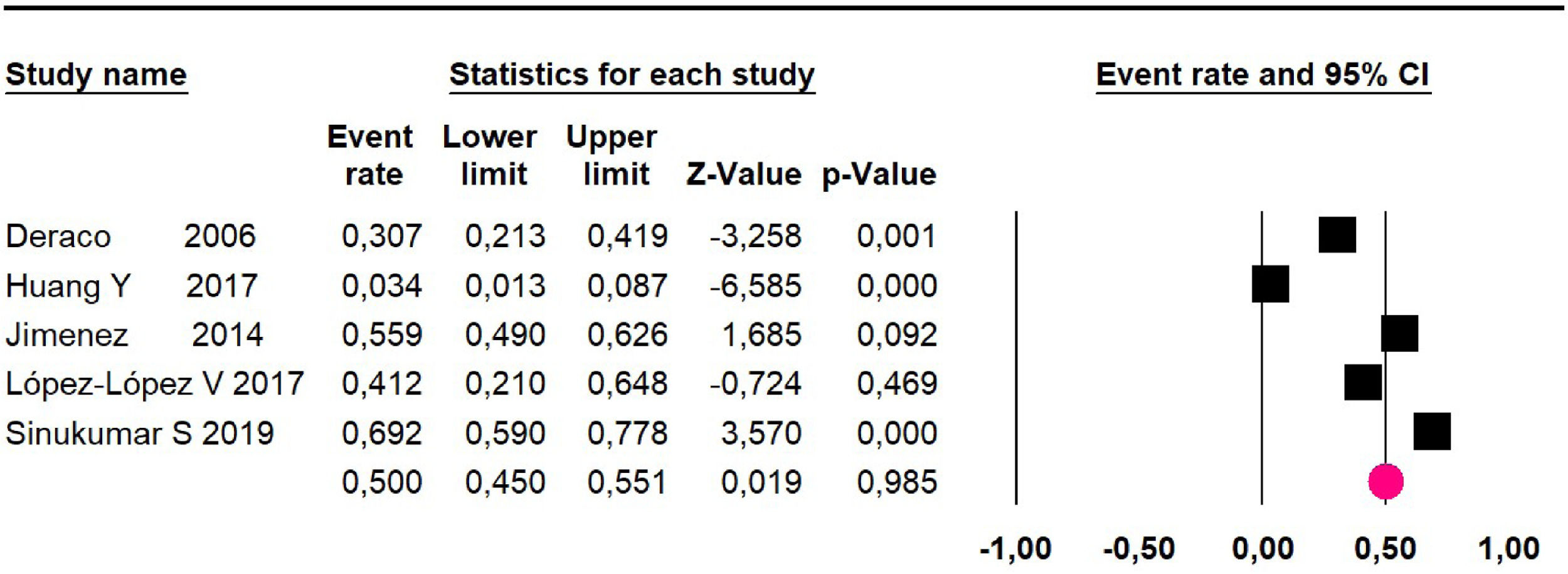
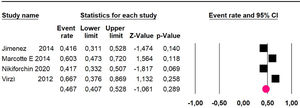
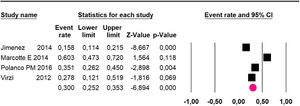
Disease-free survival: a meta-analysis of three studies,24,31,33 assessing 373 participants, the follow-up 36-month risk was 42.5% (95% CI 39.9 to 50.5; I2 = 94.13%) (Fig. 9) between participants who have undergone HIPEC and CRS.
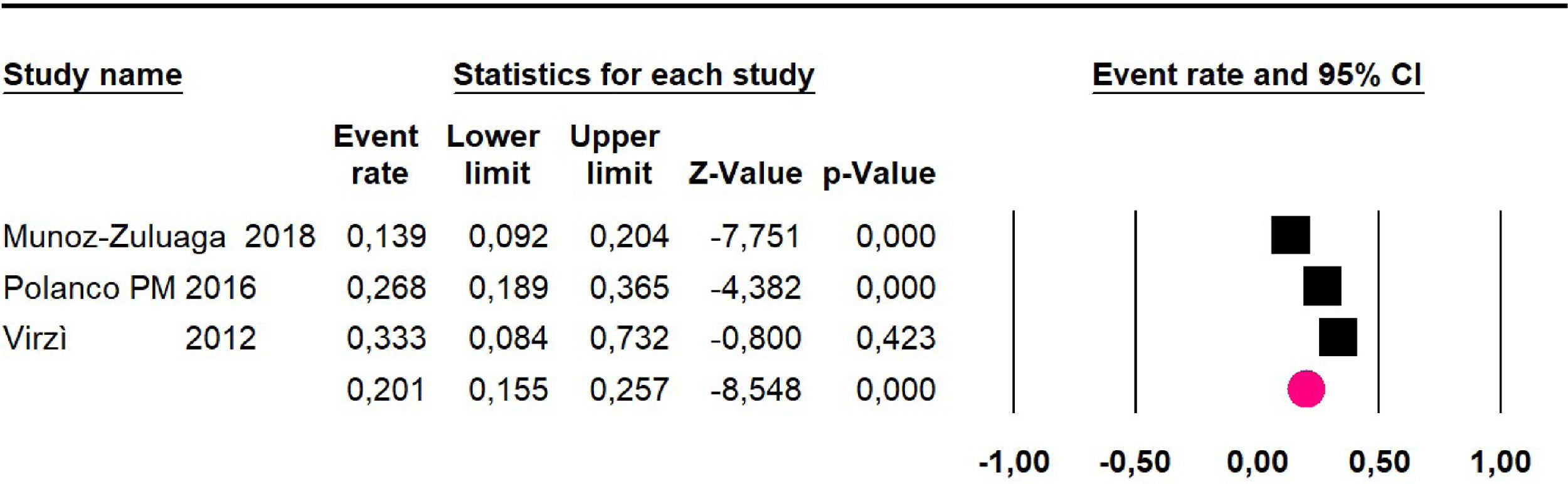
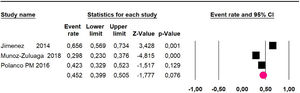
The 60-month disease-free survival: a meta-analysis of three studies31,33,39 including 254 patients, reported risk 20.1% (95% CI 15.5 to 25.7; I2 = 70.84%) (Fig. 10) between participants who have undergone HIPEC and CRS.
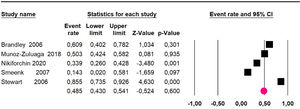
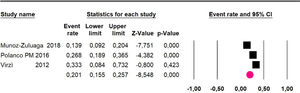
Adverse events greater than or equal to grade III: a meta-analysis of four studies24,29,33,38 assessing 375 patients, reported 60-month risk of 30% (95% CI 25.2 to 35.3; I2 = 92.8%) (Fig. 11).
Meta-analysis eighteen studies16,18-24,26-30,34,36,38-40 assessing 2594 participants evaluated HIPEC and CRS:
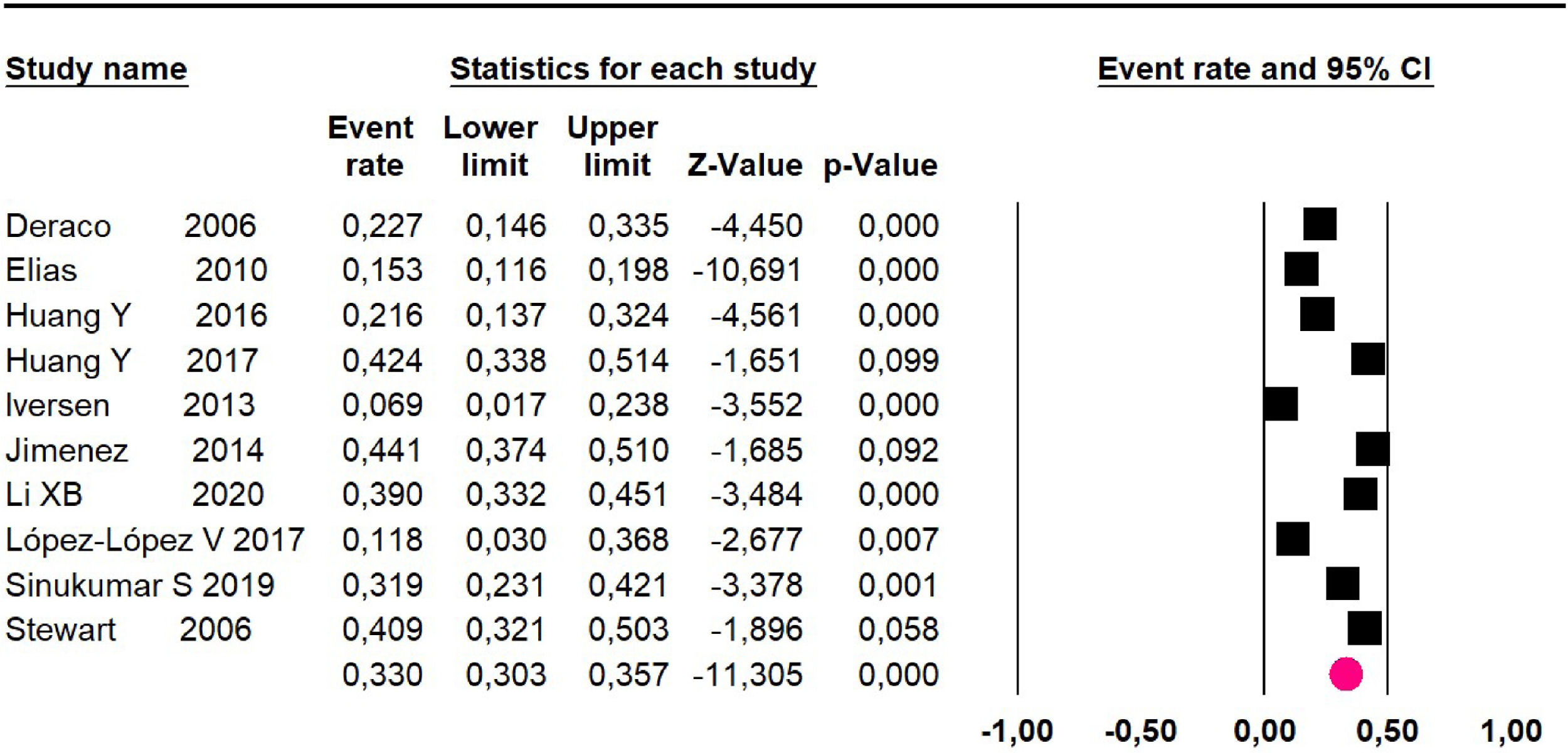
Mortality at 36-month was evaluated in ten studies18,20,21-24,26,27,34,36 including 1271 patients. The risk was 33% (95% CI 30.3 to 35.7; I2 = 88.6%) (Fig. 12).
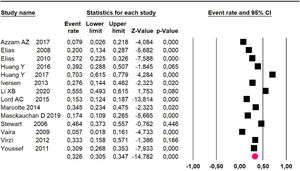
Mortality at 60-month: risk mortality was evaluated in fourteen studies13,16,17-22,25,27-29,37,39,41 [42] assessing 2209 patients, risk was 32.6% (95% CI 30.5 to 34.7; I2 = 94.45%) (Fig. 13) between participants who have undergone HIPEC and CRS.
Disease-free survival: meta-analysis of five studies18,22,24,27,34 including 503 participants, the follow-up 36-month risk was 50% (95% CI 45 to 55.1; I2 = 94.29%) (Fig. 14) between participants who have undergone HIPEC and CRS.
Disease-free survival: meta-analysis of other 9 studies16,19,20,22,28-30,37,39 including 1295 participants, reported risk of 61.8% (95% CI 58.8 to 64.7; I2 = 93.51%) (Fig. 15) at 60-month follow-up.
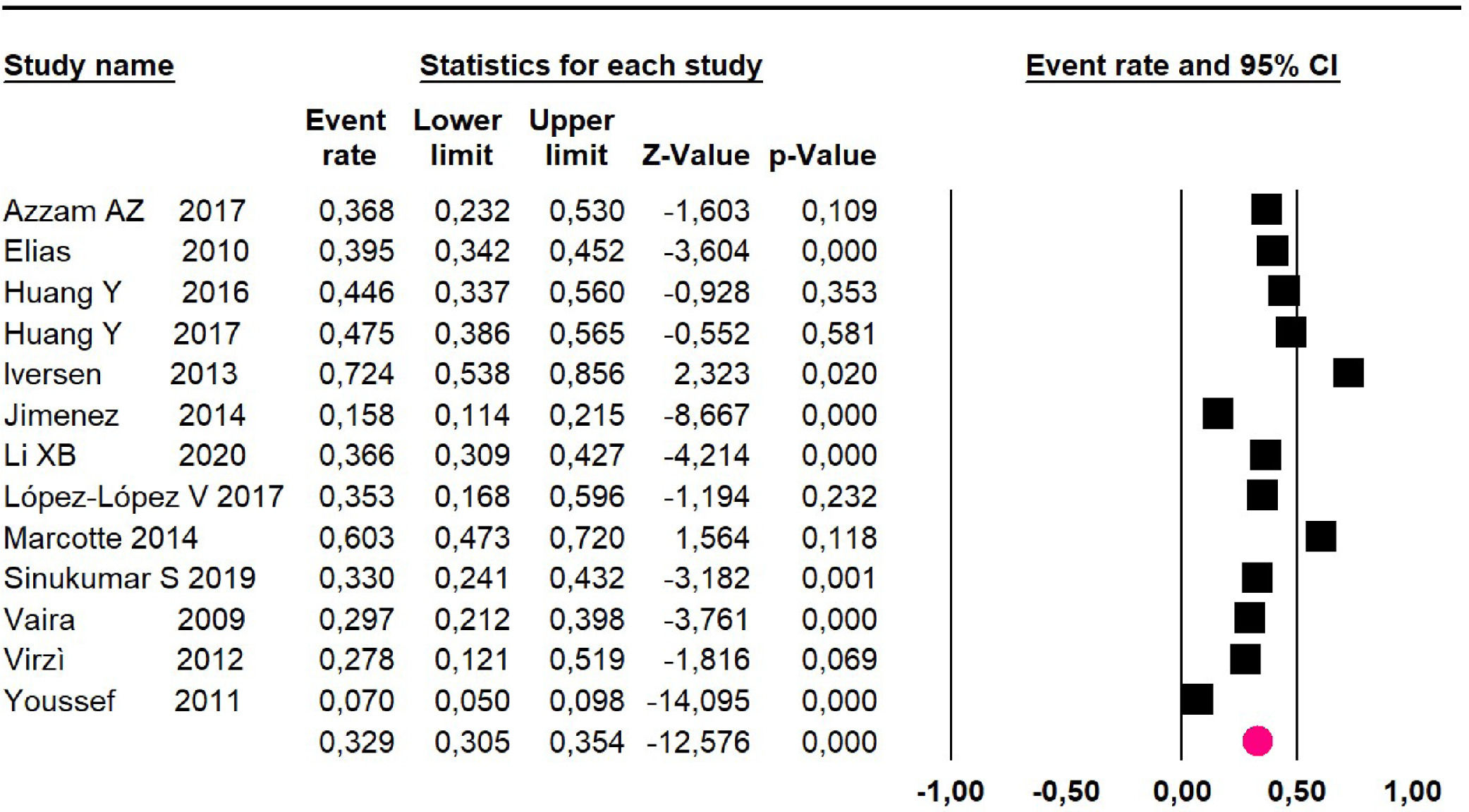
Adverse events greater than or equal to degree III: meta-analysis of 1316,20-24,26,27,29,34,38-40 studies reported adverse events to degree ≥ 3 for 1747 patients, the risk 60-month was 32.9% (95% CI 30.5 to 35.4; I2 = 93.58%) (Fig. 16).
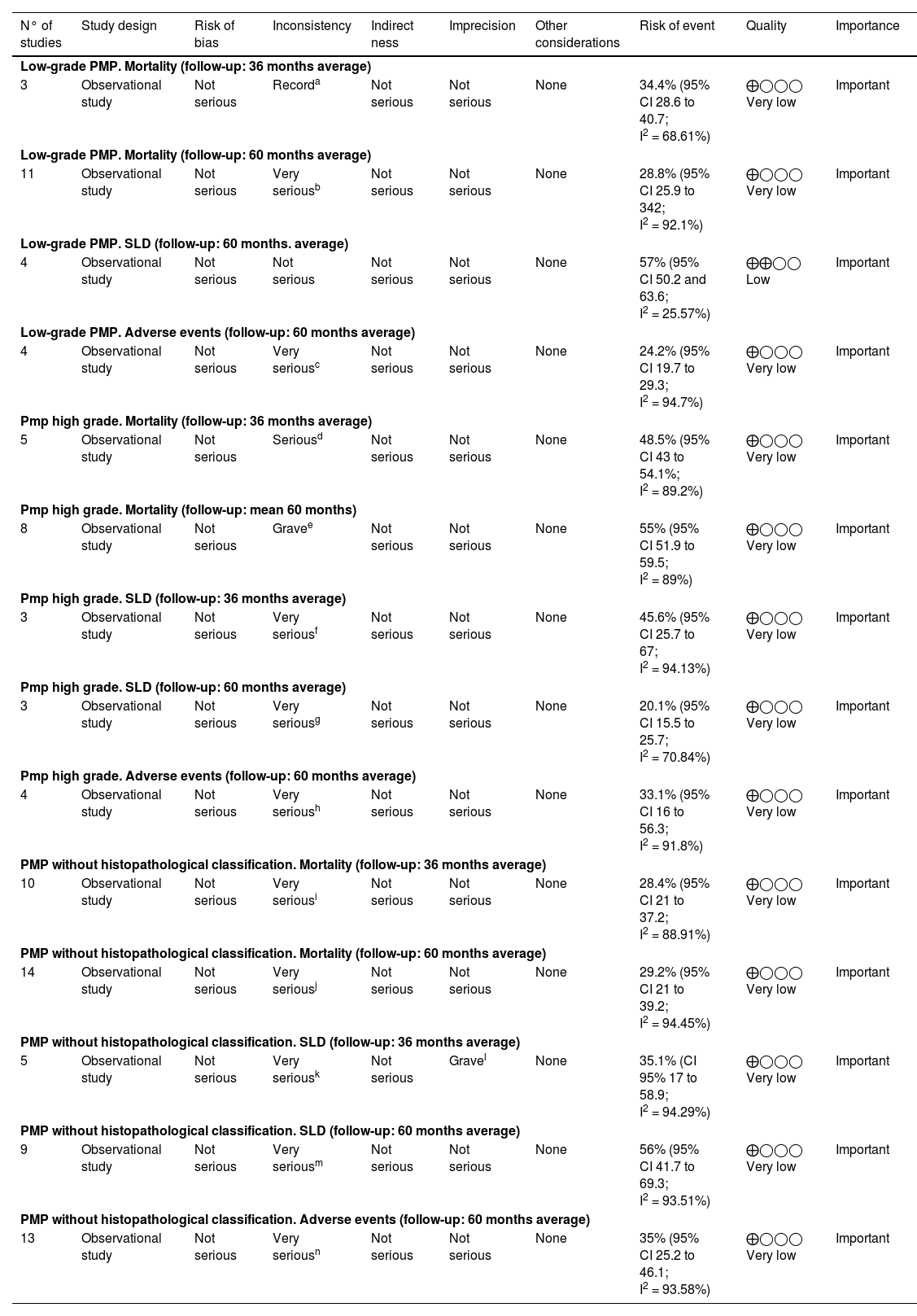
Quality of evidence was assessed using the GRADE instrument14 (Table 3) as very low quality for all outcomes, except for disease-free survival 60-month (low-grade PMP) outcome was low quality. Table 4
Summary of results and analysis of evidence GRADE.12 Peritoneal pseudomyxoma cecal appendix origin.
| N° of studies | Study design | Risk of bias | Inconsistency | Indirect ness | Imprecision | Other considerations | Risk of event | Quality | Importance |
|---|---|---|---|---|---|---|---|---|---|
| Low-grade PMP. Mortality (follow-up: 36 months average) | |||||||||
| 3 | Observational study | Not serious | Recorda | Not serious | Not serious | None | 34.4% (95% CI 28.6 to 40.7; I2 = 68.61%) | ⨁◯◯◯ Very low | Important |
| Low-grade PMP. Mortality (follow-up: 60 months average) | |||||||||
| 11 | Observational study | Not serious | Very seriousb | Not serious | Not serious | None | 28.8% (95% CI 25.9 to 342; I2 = 92.1%) | ⨁◯◯◯ Very low | Important |
| Low-grade PMP. SLD (follow-up: 60 months. average) | |||||||||
| 4 | Observational study | Not serious | Not serious | Not serious | Not serious | None | 57% (95% CI 50.2 and 63.6; I2 = 25.57%) | ⨁⨁◯◯ Low | Important |
| Low-grade PMP. Adverse events (follow-up: 60 months average) | |||||||||
| 4 | Observational study | Not serious | Very seriousc | Not serious | Not serious | None | 24.2% (95% CI 19.7 to 29.3; I2 = 94.7%) | ⨁◯◯◯ Very low | Important |
| Pmp high grade. Mortality (follow-up: 36 months average) | |||||||||
| 5 | Observational study | Not serious | Seriousd | Not serious | Not serious | None | 48.5% (95% CI 43 to 54.1%; I2 = 89.2%) | ⨁◯◯◯ Very low | Important |
| Pmp high grade. Mortality (follow-up: mean 60 months) | |||||||||
| 8 | Observational study | Not serious | Gravee | Not serious | Not serious | None | 55% (95% CI 51.9 to 59.5; I2 = 89%) | ⨁◯◯◯ Very low | Important |
| Pmp high grade. SLD (follow-up: 36 months average) | |||||||||
| 3 | Observational study | Not serious | Very seriousf | Not serious | Not serious | None | 45.6% (95% CI 25.7 to 67; I2 = 94.13%) | ⨁◯◯◯ Very low | Important |
| Pmp high grade. SLD (follow-up: 60 months average) | |||||||||
| 3 | Observational study | Not serious | Very seriousg | Not serious | Not serious | None | 20.1% (95% CI 15.5 to 25.7; I2 = 70.84%) | ⨁◯◯◯ Very low | Important |
| Pmp high grade. Adverse events (follow-up: 60 months average) | |||||||||
| 4 | Observational study | Not serious | Very serioush | Not serious | Not serious | None | 33.1% (95% CI 16 to 56.3; I2 = 91.8%) | ⨁◯◯◯ Very low | Important |
| PMP without histopathological classification. Mortality (follow-up: 36 months average) | |||||||||
| 10 | Observational study | Not serious | Very seriousi | Not serious | Not serious | None | 28.4% (95% CI 21 to 37.2; I2 = 88.91%) | ⨁◯◯◯ Very low | Important |
| PMP without histopathological classification. Mortality (follow-up: 60 months average) | |||||||||
| 14 | Observational study | Not serious | Very seriousj | Not serious | Not serious | None | 29.2% (95% CI 21 to 39.2; I2 = 94.45%) | ⨁◯◯◯ Very low | Important |
| PMP without histopathological classification. SLD (follow-up: 36 months average) | |||||||||
| 5 | Observational study | Not serious | Very seriousk | Not serious | Gravel | None | 35.1% (CI 95% 17 to 58.9; I2 = 94.29%) | ⨁◯◯◯ Very low | Important |
| PMP without histopathological classification. SLD (follow-up: 60 months average) | |||||||||
| 9 | Observational study | Not serious | Very seriousm | Not serious | Not serious | None | 56% (95% CI 41.7 to 69.3; I2 = 93.51%) | ⨁◯◯◯ Very low | Important |
| PMP without histopathological classification. Adverse events (follow-up: 60 months average) | |||||||||
| 13 | Observational study | Not serious | Very seriousn | Not serious | Not serious | None | 35% (95% CI 25.2 to 46.1; I2 = 93.58%) | ⨁◯◯◯ Very low | Important |
IC; Confidence Interval; I2 heterogeneity.
Explanations:
Synthesis of evidence.
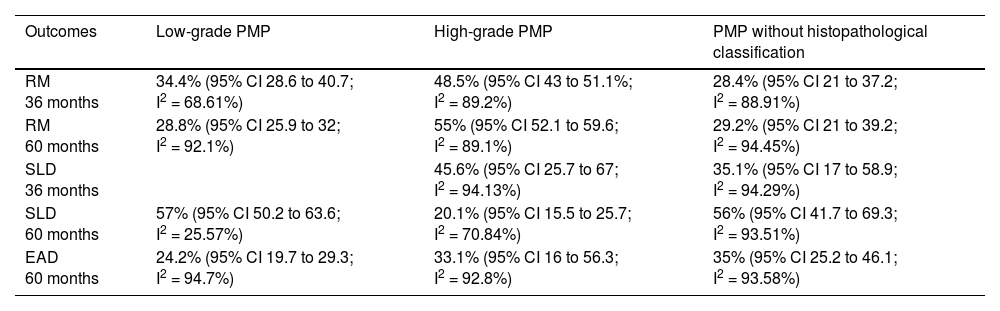
| Outcomes | Low-grade PMP | High-grade PMP | PMP without histopathological classification |
|---|---|---|---|
| RM 36 months | 34.4% (95% CI 28.6 to 40.7; I2 = 68.61%) | 48.5% (95% CI 43 to 51.1%; I2 = 89.2%) | 28.4% (95% CI 21 to 37.2; I2 = 88.91%) |
| RM 60 months | 28.8% (95% CI 25.9 to 32; I2 = 92.1%) | 55% (95% CI 52.1 to 59.6; I2 = 89.1%) | 29.2% (95% CI 21 to 39.2; I2 = 94.45%) |
| SLD 36 months | 45.6% (95% CI 25.7 to 67; I2 = 94.13%) | 35.1% (95% CI 17 to 58.9; I2 = 94.29%) | |
| SLD 60 months | 57% (95% CI 50.2 to 63.6; I2 = 25.57%) | 20.1% (95% CI 15.5 to 25.7; I2 = 70.84%) | 56% (95% CI 41.7 to 69.3; I2 = 93.51%) |
| EAD 60 months | 24.2% (95% CI 19.7 to 29.3; I2 = 94.7%) | 33.1% (95% CI 16 to 56.3; I2 = 92.8%) | 35% (95% CI 25.2 to 46.1; I2 = 93.58%) |
RM, Mortality risk; EAD, Adverse Events.
Low-grade PMP: mortality risk follow-up 36-month, 60-month, DFS 60-month, adverse events to degree ≥ 3 in 60-month follow-up risk was: 34.4% (95% CI 28.6 to 40.7; I2 = 68.61%); 28.8% (95% CI 25.9 to 32; I2 = 92.1%), 57% (95% CI 50.2 to 63.6; I2 = 25.57%) and 24.2% (95% CI 19.7 to 29.3; I2 = 94.7%).
High-grade PMP: mortality risk follow-up 36-month, 60-month, DFS 36-month, DFS 60-month, adverse events to degree ≥ 3 in 60-month follow-up risk was: 48.5% (95% CI 43% to 54.1%, I2 = 89.2%), 55.9% (95% CI 52.1 to 59.6; I2 = 89.1%), 45.6% (95% CI 25.7 to 67; I2 = 94.13%), 20.1% (95% CI 15.5 to 25.7; I2 = 70.84%); and 33.1% (95% CI 16 to 56.3; I2 = 92.8%).
PMP without histopathological classification: mortality risk follow-up 36-month, 60-month, DFS 36-month, DFS 60-month, adverse events to degree ≥ 3 in 60-month follow-up risk was: 28.4% (95% CI 21 to 37.2; I2 = 88.91%), 29.2% (95% CI 21 to 39.2; I2 = 94.45%), 35.1% (95% CI 17 to 58.9; I2 = 94.29%), 56% (95% CI 41.7 to 69.3; I2 = 93.51 and 35% (95% CI 25.2 to 46.1; I2 = 93.58%).
DiscussionThe absence of randomized and controlled studies results in the low incidence of the disease, 0.2 to 2 cases per 1.000.000 inhabitants per year.41 In the present systematic review, with meta-analysis, the authors found only a series of cases, the fact that compromises the quality of the evidence presented.
Historically the prognosis of peritoneal pseudomyxoma is associated with origin (ovary, mesus, uric, stomach, colon, and appendix), and Cytological grading of malignancy (adenomatous, carcinomatous, and intermediate) and peritoneal dispersion index.5
Currently, the treatment is performed through peritoneal cytoreduction with or without intrabdominal hyperthermic chemotherapy.
When the authors meta-analyze the low-grade PMP outcomes without histopathological classification, in 36-months, there was an observed improvement in survival for patients without histopathological classification, but in a 60-month outcome, there is a significant improvement in low-grade PMP patients; it can be justified by the slow progression of the disease in low-grade PMP in relation to high-grade, and it may increase the mortality in this group, reducing long-term survival.
When comparing DFS in the low-grade PMP groups and those without histopathological classification, in 60-months, the authors observed similar results, 57% and 56%, a fact that can be explained by the survival of patients with better surgical results, who are better likely to remain disease-free.
The studies evaluated individually present great differences between themselves, such as Masckauchan et al.,30 which reported a result of 0% in the mortality of patients with low-grade PMP in 60-months, while Smeenk et al.,35 presented mortality of 34% of the patients. This important variation between the results may be correlated with the sample number, the chemotherapeutic drug used, the clinical and demographic characteristics of patients, surgical classification, and experience of the surgical team in the execution of the procedure.
Currently, there are difficulties in commercializing mitomycin chemotherapeutic drugs, being the most used for the execution of HIPEC. Marcotte et al.29 and Masckauchan et al.30 analyzed the survival of patients with PMP submitted to CRS and HIPEC with oxaliplatin, chemotherapy of the same family as cisplatin and carboplatin, obtaining results similar to mitomycin, and therefore, it can be used during the HIPEC procedure.
ConclusionPeritoneal polymyxoma of the appendix is a rare disease with slow evolution and survival that depends on factors such as histological degree, peritoneal cytoreductive surgery and experience of the surgical team. Hyperthermic chemotherapy is recommended in selected cases with satisfactory results.
Authors' contributionsIdevaldo F, Antonio S and Wanderley MB designed the study, performed the data collection and analysis, and critically reviewed the final version of the manuscript. João CR and Claudia C acquired some of the data. All authors read and approved the final version of the manuscript.
This review was carried out by the Evidence-Based Medicine Center, supported by the Unimed Medical Cooperative of Baixa Mogiana, Mogi-Guaçu/SP, and Federation of the Unimed of The State São Paulo (FESP) SP, Brazil.