The objective of this retrospective study is to analyze and compare the results of conventional surgical repair and endovascular treatment of blunt aortic injury over the past 8 years.
METHODSTwenty-eight patients (25 male; mean age, 35 years) were treated for blunt aortic injury between April 2001 and March 2009 in a university hospital in Brazil. Twenty-six patients were included in the study: five were treated with operative repair (OR) and 21 with endovascular treatment (TEVAR). Two patients were excluded from analysis: one was managed conservatively, and one was treated with endovascular treatment for chronic dissection related to aortic trauma.
RESULTSMean age was lower in the OR group than in the endovascular treatment group (17.8 vs. 38 years, P = .003). There was one death in the OR group and four deaths in the endovascular treatment group. Mean follow-up for the overall group was 33.6 months, with 48.7 months (range 8-83 months) for the OR group, and 29.8 months (range 2-91 months) for the TEVAR group. Mean time elapsed from injury to repair was 23.4 hours (range 8-48 h, median 20 h) for the OR group and 30.3 hours (range 2-240 h, median 18 h) for the TEVAR group (P = .374). The duration of surgery was shorter in the endovascular treatment group (142 versus 237 minutes; P = .005). There were no significant differences with respect to the number of postoperative days requiring mechanical ventilation, duration of ICU stay or duration of hospital stay.
CONCLUSIONIn this retrospective analysis, endovascular treatment was a safe method for repair of blunt aortic trauma, with immediate and midterm results that were comparable to those results obtained with operative repair. No complications from the stent graft were identified during follow-up. Nevertheless, long-term follow-up is necessary to confirm the effectiveness of this treatment.
Blunt thoracic aortic injury is associated with a high mortality rate. It is a devastating consequence of deceleration trauma. Aortic injury is second only to head injury as the leading cause of death from vehicle crashes.1,2 More than 80% of patients die at the scene, and most of those who survive the initial injury will die without definitive treatment.2,3
The force that causes the aorta to tear often leads to other organ injuries. Up to 25% of patients with blunt aorta injury require a major operation before aortic repair.4,5 Therefore, definitive treatment is often delayed due to these multiple concomitant injuries, an aspect that accounts for an in-hospital rupture rate of 10 to 13%, usually within a few hours after admission.3,6,7 The aortic tear occurs most often at the aortic isthmus, and, in order of frequency, affects the proximal descending aorta, the ascending aorta, the aortic arch, distal descending aorta, and the abdominal aorta.4,8
Traditional treatment of blunt aortic injury has been early open surgical repair with graft interposition, with or without adjuncts to maintain distal perfusion.2,4,9 Open repair carries a 2.9 to 7% risk of paraplegia and an operative mortality rate ranging from 15 to 23.5%.4,9–11 Nevertheless, the introduction of endovascular stent grafts is revolutionizing the definitive treatment of these injuries.11 Although endovascular management of aortic rupture was initially restricted to high-risk patients with multiple injuries, in many centers it has now become the preferred first treatment even in young or low-risk patients.11 The potential benefits of thoracic endovascular aortic repair (TEVAR) over open repair include no need for thoracotomy or single lung ventilation, decreased use of systemic anticoagulation, avoidance of aortic cross-clamping, less blood loss, less postoperative pain and lower paraplegia rate.3 These factors can improve overall survival as documented by several meta-analyses.4,9,10 At our institution we have been performing TEVAR to treat blunt aortic injury since 2001.
The purpose of this retrospective study was to compare the results of conventional surgical repair and endovascular treatment of blunt aortic injury performed in one single institution, a university hospital in Brazil. We present our midterm results and analyze the technical challenges we have faced in 8 years of experience. We believe this report represents one of the largest single-center experiences with endograft repair of blunt aortic injury published to date,2,4,9 and the most expressive in South America.
MATERIALS AND METHODSBetween April 2001 and March 2009, 28 consecutively patients with acute traumatic rupture of the descending aorta received treatment at our institution. Eighteen patients were admitted directly to our trauma center, and 10 patients were referred from secondary trauma centers. Twenty-five patients had traumatic injury involving sudden deceleration (4 falls from great height, 21 road accidents) and 3 patients were hit by vehicle.
The group included 3 female and 25 male patients, aged 11 to 72 years (mean of 35 years). In all patients diagnosis was made by computed angiotomography (CT). CT is liberally indicated in patients who sustain a mechanism of severe trauma, as well as in patients with clinical signs or findings on chest x-ray.
One patient with minimal aortic injury was treated conservatively and excluded from the series. Another patient received endovascular treatment for chronic dissection related to trauma and was also excluded from acute trauma data analysis. The remaining 26 patients were divided into 2 groups: operative repair (OR, 5 patients) and endovascular repair (TEVAR, 21 patients). The type of treatment adopted, initially depended on the severity of trauma and the availability of stents graft, with TEVAR reserved for high-risk patient. However, with the growing experience of the surgical team, TEVAR replaced open surgery as the first line treatment at our institution. All data were collected by analyzing medical reports and information in outpatient care. However, due to the retrospective aspect of this study, an informed consent was not provided to the patients.
Data collectionPreoperative variables included age, gender, date of injury and diagnosis, mechanism and type of injury, x-ray findings, associated injuries, Glasgow coma scale (GCS), admission vital signs (heart rate, blood pressure), creatinine, need for blood transfusion, injury severity score (ISS, ranging from 0 to 75), revised trauma score (RTS, normal score = 7.84), and trauma injury severity score (TRISS – prediction of death rate).12 The time elapsed from trauma to initial care and admission to intervention was also recorded.
The following operative variables were recorded: type of procedure, duration of procedure, use of systemic anticoagulation, use of circulatory support, intraoperative blood and plasma transfusion. Specific to TEVAR, the number and type of stent graft, the access and complications, covering of left subclavian artery, the use of balloon, and identification of endoleak were also recorded. Postoperative variables included mortality, paraplegia, need for re-intervention, procedure-related complications, clinical complication, changes in creatinine, duration of mechanical ventilation, intensive care unit (ICU) and hospital length of stay, CT control and clinical follow-up.
Surgical techniqueOpen repair (OR) was performed under general anesthesia. A left posterolateral thoracotomy was used in all cases of open repair. The repair technique included interposition grafts in all 5 patients. Extracorporeal circulatory support (atrio-femoral bypass) was performed in 3 patients.
Endovascular repair was performed in the operating room under general anesthesia. Access was obtained primarily via the common femoral arteries; in 1 patient the approach was via the common iliac artery due to the diameter of the femoral artery. All TEVAR procedures were performed by the vascular surgery team in the operating room with portable C-arm fluoroscopy (BV Pulsera; Phillips). The grafts were oversized by 10 to 25%. We implanted five different commercially available thoracic stent grafts: 1 Apolo (Nano, SC, Brazil), 1 Excluder (W. L. Gore & Associates, USA), 3 Zenith (Cook, Australia), 5 Talent (Medtronic, Santa Rosa, CA, USA) and 12 Valiant (Medtronic, Santa Rosa, CA, USA). Diameters ranged from 24 to 34 mm, and length from 100 to 200 mm. A catheter used for angiography during graft deployment was placed percutaneously through the contralateral femoral artery. In 17 patients, the rupture was located near or distal to the origin of the left subclavian artery; in 1 patient, the lesion was at the distal thoracic aorta; 2 patients had a rupture associated with limited dissection and 1 patient had a type B aortic dissection. A CT scan was performed prior to discharge, and follow-up imaging was planned after 3 and 6 months, and yearly thereafter.
Statistical analysisStatistical analysis comparing groups 1 and 2 was carried out with SPSS (version 10.0; SPSS, Chicago, ILL). The Mann-Whitney U test was used to compare values with non-normal distribution. Nominal variables were compared using the Fisher exact test. P < .05 was chosen to denote statistical significance.
RESULTSOver 8 years, 26 patients were treated with OR or TEVAR for acute thoracic aortic rupture (mean of 3.5 patients per year) at our institution. Five patients were treated with OR vs. 21 treated with TEVAR. There were no significant differences between TEVAR and OR with respect to preoperative comorbidity and variables on admission (Table 1), with the exception of mean age, which was lower in the OR than in the TEVAR group (17.8 vs. 38 years, P = .003).
Preoperative comorbidity and variables on admission.
| Preoperative variable | OR (n = 5) | TEVAR (n = 21) | P value |
|---|---|---|---|
| Mean age in years | 17.8 | 38.1 | .003* |
| Mean RTS | 7.25 | 7.08 | .81 |
| Mean ISS | 39 | 46.7 | .39 |
| Mean TRISS | 15.5 | 31.1 | .28 |
| Mean GCS | 13 | 12.5 | .76 |
| Mean creatinine (preprocedure) | 0.86 | 1.07 | .13 |
| Mean time to initial care, minutes | 39 | 42.9 | .75 |
| Mean time to repair, hours | 23.4 | 30.3 | .37 |
| Transferred from secondary trauma center (%) | 1 (20%) | 10 (48%) | .61 |
Note: OR, Open repair; TEVAR, Thoracic endovascular aortic repair; RTS, revised trauma score; ISS, injury severity score; TRISS, trauma injury severity score. *Significant at P <.05.
The mean time elapsed from injury to repair was 23.4 hours (range 8-48 h, SD: 15.4) for the OR group and 30.3 hours (range 2-240 h, SD: 54.3) for the TEVAR group (P = .374). In all 26 patients angiotomography was used for diagnosis and surgical planning. The use of CT positively impacted diagnostic precision and the feasibility of endovascular treatment of aortic trauma.
Four patients in the OR group received initial care at our institution and 1 case was referred from another center, while 10 cases in the TEVAR group had initial care at secondary centers and were then referred to our level I trauma center.
Open repair groupAll 5 patients underwent repair under general anesthesia with single lung ventilation through a left posterolateral thoracotomy. Three patients required circulatory support. The damaged portion of aorta was replaced with a Dacron® prosthetic graft in all 5 patients. One patient died right after surgery due to coagulopathy and multiple organ failure. Heparin was used in four cases without complications. No postoperative paraplegia occurred in this group.
Endovascular groupAll 22 endovascular repairs were performed under general anesthesia. One patient had a lesion of the right common femoral artery, corrected with a PTFE graft. Nineteen procedures were carried out with successful deployment of a single endograft; in two cases, a proximal extension was required. There was no procedure-related paraplegia, and no conversions to open repair. We covered the ostium of the left subclavian artery in 9 cases (42.8%), because of insufficient length of the proximal neck, with unremarkable clinical impact.
Intraoperative results for the two groups are summarized in Table 2.
Comparison between operative variables.
| Intraoperative variable | OR (n = 5) | TEVAR (n = 21) | P value |
|---|---|---|---|
| Mean operative time, min | 237 | 142 | .005* |
| Heparinized, n (%) | 4 (80) | 7 (33) | 1.0 |
| Mean PRBC, U | 6.4 | 3.4 | .054 |
| Mean FFP, U | 2 | 0.5 | .031* |
Note: PRBC, packed red blood cells; FFP, fresh frozen plasma. *Significant at P < .05.
Statistically significant difference was observed only for operative time, with shorter duration in the TEVAR group (142 versus 237 minutes; P = .005).
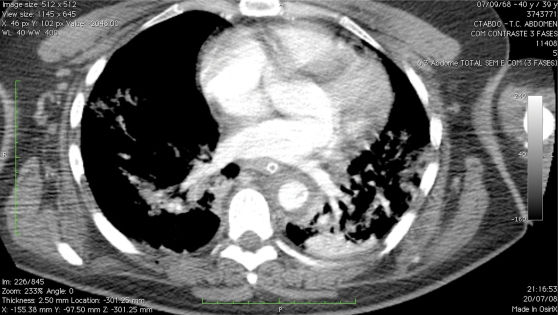
There were four deaths in the TEVAR group (19.4%) (Table 3). One patient had rupture of the aortic lesion (grade IV)13 prior to surgery and was promptly treated, but died in the ICU due to serious coagulopathy, hypotension and hypothermia less than 24 hours after the surgery (Figures 1 and 2). One patient had associated abdominal aorta lesion, and died in the ICU 18 hours after the surgery because of refractory shock and coagulopathy. One patient died in the ICU due to abdominal sepsis associated with acute gastrointestinal hemorrhage on postoperative day 19. The last patient (TRISS 93.4%) was submitted to three operative procedures (drainage of subdural hematoma, endovascular repair of aortic lesion, and laparotomy to treat bleeding due to grade III renal injury) and died in the ICU less than 24 hours after surgery.
Outcomes.
| OR (n = 5) | TEVAR (n = 21) | P value | |
|---|---|---|---|
| Mortality at 30 days (%) | 1 (20) | 4 (19,04) | 1.0 |
| Treatment-related paraplegia | 0 | 0 | - |
| Mean ventilator days (+/- SD)* | 4.7±3.0 | 10.3±14.9 | .79 |
| Mean ICU days (+/- SD)* | 12.5±10.7 | 18.6±17.3 | .73 |
| Mean hospital stay in days (+/- SD)* | 22.0±10.8 | 31.7±40.2 | .93 |
| Mean follow-up in months (+/- SD) | 48.7 (35.3) | 29.8 (28) | .40 |
| Endoleak / Migration | N/A | 0 | - |
Note: *patients who died in the first 24 hours (n = 4) were excluded; SD, Standard deviation; ICU, intensive care unit; N/A, not applicable
There were no significant differences between the groups with respect to the number of postoperative days requiring mechanical ventilation, duration of ICU stay or duration of hospital stay (Table 3).
Follow-upMean follow-up for the overall group was 33.6 months (range 2-91 months). In the OR group, mean follow-up was 48.7 months (range 8-83 months). Two patients were lost to follow-up 8 and 31 months after surgical treatment. During follow-up, 1 patient with stenosis of proximal anastomosis was treated clinically.
In the TEVAR group, mean follow-up was 29.8 months (range 2-91 months). Two patients were lost to follow-up 3 and 5 months after surgical treatment. No endoleak, migration or damage to the stent-graft material were detected (Figure 3).
We had one case of migration of the uncovered stent (free-flow) into the left subclavian artery (Figure 4). The patient has remained asymptomatic for 61 months.
Minimal aortic injuryWe had one female patient with intimal flap associated with periaortic hematoma. She had severe trauma (ISS 34 and TRISS 11.3) and was followed closely in the ICU with serial CT scans. The lesion was healed at 8 weeks (Figures 5 and 6).
Chronic aortic dissectionOne of our patients was a 52-year old male patient who was initially treated conservatively by another vascular surgery group. We decided for endovascular correction based on good clinical condition and relatively young age. He was successfully treated 5 months after trauma with a Zenith endograft (Cook, Australia) 30 × 200 mm + Zenith Dissection TXD 46 × 164 mm (Figure 7).
DISCUSSIONBlunt aortic injury is still a major cause of death, leading to 7500-8000 deaths per year in the USA.2,9,14 For those who arrive alive, the overall mortality is about 32%.2-9,11 These statistics indicate the need for early diagnosis and urgent surgical treatment. The management of these patients includes careful monitoring and control of blood pressure and pulse rate (beta-blockers).3,7 Several studies demonstrate relative safety for a delayed approach,7,15 especially after the introduction of beta blockers, which have reduced the risk of in-hospital free rupture.7 However, patients with no substantial associated injuries are preferentially submitted to emergency surgery.2,7
In recent years, an increasing number of blunt aortic injury patients has been treated with TEVAR. At our institution, conventional surgical repair is no longer the procedure of choice. In 2001, only our high-risk patients were treated with the endovascular approach; however, with the development of new aortic stent grafts and the growing experience of the team, the number of patients treated with TEVAR increased up to a point where TEVAR replaced open surgery as the first line treatment for blunt aortic injury at our institution. Open surgery was last used by us to treat a patient in 2004 (Chart 1).
During our 8-year experience with TEVAR, we have observed a similar rate of perioperative complications and mortality as compared to OR. The fact that the TEVAR group was significantly older than the OR group, with more severe mean trauma scores than the OR group (although without statistical significance), further supports the safety of TEVAR relatively to OR. The mean predicted death rate (TRISS score) for TEVAR was 31.1% vs. 15.5% for OR (P = .28).
Our trauma center provides care to patients referred from many other institutions in the city of São Paulo, especially for critical cases. Because of the structure of the Brazilian healthcare system, some patients were delayed in reaching our institution. We believe that the time elapsed between trauma and specific care is essential for a successful treatment. Mean time from injury to repair in patients receiving treatment directly at our service was 19 hours, as compared to 45.5 hours in patients receiving initial care at another institution (P = 0.343).
In our series, we had only two procedure-related complications in the TEVAR group. There was one case of injury during the access of the common femoral artery, immediately repaired with a PTFE graft. With respect to the endograft, we had 1 patient with a bare metal stent (free-flow) covering the origin of the left subclavian artery (Figure 4). This patient is being closely monitored, with unremarkable clinical impact. The left subclavian artery was covered in 9 cases (42.8%) due to insufficient length of the proximal neck. Although no vascular study of vertebral system was performed during surgery, there was no neurological complication at follow-up.
We used 5 different stent grafts in 22 patients (24- 34 mm in diameter), with maximal oversizing of 25%. No migration or endoleak have been detected during follow-up.
Procedure-related complications as a direct result of TEVAR have been previously reported, including endoleaks, stent fracture, partial and complete stent collapse, stroke, need for conversion to open repair and stent migration.3,4,9–11,16-26 This can be partially explained by the fact that the endografts used to treat blunt trauma have been primarily designed for aortic aneurysms. The trauma population is younger, with a smaller aortic diameter than that of the population of patients receiving TEVAR for aneurysmatic disease (the average diameter in trauma patients is 19 mm).2,11 Attempts to place an endograft in aortas with less than 23 mm in diameter can create a situation of significant oversizing and have been associated with device collapse.16,17 Another issue that is often neglected concerning excessive oversizing in young patients refers to the possible harmful impact on the aortic wall with the passing of time. Not only is the young aorta narrower, it also has a much tighter turn radius of the aortic arch. As previously stated, injuries occurring next to a sharp bend in the aorta can compromise apposition of the covered stent to the aortic wall,2 which in turn can lead to failure in covering the injury. Such malpositioning, especially when associated with excessive oversizing, can also cause collapse of the endograft. The development of curved stent grafts, with smaller diameters devices and lower profiles can improve the results of TEVAR for blunt aortic trauma.
In 14 patients of the TEVAR group, systemic anticoagulation was contraindicated due to serious head and other injuries. We had no thrombotic or embolic complications using local heparinization. The 7 patients who received systemic anticoagulation had no hemorrhagic complications. Systemic heparinization was not used in the OR group in 1 patient with severe injuries who developed coagulopathy. Unfortunately, this patient did not survive.
Regarding blood administration, although some retrospective studies have demonstrated decreased use of blood products with TEVAR,19 it is difficult to discern the need for blood because these patients have multiple associated injuries. In our series, the TEVAR group had a mean intraoperative administration of packed red blood cells of 3.4 compared to 6.4 for the OR group (P = .057). There was also a trend towards decreased administration of fresh-frozen plasma (mean of .5 UI for TEVAR vs. 2 UI for OR – P = .121). We noticed a significant decrease in operating room time for TEVAR (Table 2). We believe that reducing operating room time is important to decrease the systemic inflammatory response and the exposure to low temperatures. In addition, it might enable a combined therapeutic strategy in selected cases.
In our series, no cases of paraplegia were observed. Two important meta-analyses of studies comparing TEVAR to OR revealed that the risk of paraplegia is significantly lower with endovascular management (0% vs. 5.6-7%).4,9 Although the studies analyzed were heterogeneous with respect to type of endovascular stent-graft and timing of intervention, all reported more favorable early and midterm results following endovascular repair, including decreased mortality. The overall 30-day mortality in theses meta-analyses was lower after endoluminal repair (7.6% and 8%) compared with open repair (15.2% and 20%).4,9 A survival benefit and lower paraplegia rate for TEVAR have also been reported by the American Association for the Surgery of Trauma based on a prospective multicenter study.11 In our cohort, overall 30-day mortality was 19% for TEVAR and 20% for OR (P = 1.0, Table 3). Although the present population is too small for generalization of the results, we believe that the observed TEVAR-related mortality was strongly influenced by the pre-treatment clinical status of the patients in this group.
There were no midterm complications with TEVAR during a mean follow-up of 29.8 months (range 2-91). Lifelong clinical and imaging follow-up are indicated for trauma patients to ensure detection of graft failure.
We describe one case of minimal aortic injury, which serves to discuss an issue raised by Professor Mattox11 – that in published series of aortic trauma “at least one case, and probably more… had a CT scan with an almost non-injury aorta, and a stent graft was deployed as well.” We agree that in such situations unnecessary endografts may end up being inserted. However, it is very difficult to establish an ideal criterion to determine which intimal lesions demand treatment. Our institution is very conservative with placement of stent grafts, due to the training of our surgeons and the financial burden that an endograft represents in a developing country. Angiotomography is now capable of identifying many more injuries than in the past. A reasonable number of patients whose aortic injury would have been missed in the past can now benefit from a precise diagnostic and therapeutic strategy. Moreover, patients with minimal injuries who did not have a choice of surgery are now offered endovascular treatment. It is very important to avoid excess, with treatment being indicated strictly in cases of aortic “intimal tear” with significant risk of rupture (tear > 1 cm associated with hematoma or dissection). Clinical status must also be taken into consideration. Intravascular ultrasound is very useful to define the best management.13 A recent classification of aortic injury (grades I-IV) proposed by Azizzadeh et al.,13 based on angiotomography and intravascular ultrasound can be very helpful for decision-making.13
With respect to chronic dissection associated with trauma, very little data are available in the literature. Our first option is to treat acutely so as to prevent further complications. The diagnosis of chronic dissection is often questionable unless in the presence of a direct association with the trauma and a diagnostic image of the aortic injury. We believe that an early attempt to treat this dissection would allow reapproximate the true and false lumen, and theoretically prevent aortic dilatation.
During eight years using TEVAR to treat blunt aortic trauma, we have used five different thoracic endoprostheses. We have made several changes in the management of these patients, in search of the best commercial available endograft for aortic trauma. We believe that the ideal endograft should be designed specifically for trauma patients with the following features: absence of free-flow or bare stent; maximum length of 10 cm; oversizing very close to 10% but not more than 20%; proximal curvature in aortic arch angle.
Limitations of our series include its retrospective nature, the heterogeneity of the groups and the variety of stent grafts. It is difficult to generate enough power to reveal significant differences due to the small size of the study population.
CONCLUSIONThis retrospective study shows that endovascular treatment is a safe method for repair of blunt aortic trauma, with immediate and midterm results that are at least comparable to those obtained with operative repair. No definitive conclusions can be drawn from these observational studies, but the results seem to indicate the feasibility and safety with TEVAR. Endovascular management presents lower morbidity, precludes the use of aortic cross-clamp, allows simultaneous treatment of associated injuries, and can be safely done without use of systemic heparin. Although TEVAR has become the first line therapy at several institutions, long-term follow-up is necessary to determinate the real effectiveness of this treatment. The development of new stent-grafts designed specifically for trauma injuries will benefit a larger number of patients.