The antibacterial effect of ozone (O3) has been described in the extant literature, but the role of O3 therapy in the treatment of certain types of infection remains controversial.
OBJECTIVESTo evaluate the effect of intraperitoneal (i.p.) O3 application in a cecal ligation/puncture rat model on interleukins (IL-6, IL-10) and cytokine-induced neutrophil chemoattractant (CINC)-1 serum levels, acute lung injury and survival rates.
METHODSFour animal groups were used for the study: a) the SHAM group underwent laparotomy; b) the cecal ligation/puncture group underwent cecal ligation/puncture procedures; and c) the CLP+O2 and CLP+O3 groups underwent CLP+ corresponding gas mixture infusions (i.p.) throughout the observation period. IL-6, CINC-1 and IL-10 concentrations were determined by enzyme-linked immunosorbent assay (ELISA). Acute lung injury was evaluated with the Evans blue dye lung leakage method and by lung histology. P<0.05 was considered significant.
RESULTSCINC-1 was at the lowest level in the SHAM group and was lower for the CLP+O3 group vs. the CLP+O2 group and the cecal ligation/puncture group. IL-10 was lower for the SHAM group vs. the other three groups, which were similar compared to each other. IL-6 was lower for the SHAM group vs. all other groups, was lower for the CLP+O3 or CLP+O2 group vs. the cecal ligation/puncture group, and was similar for the CLP+O3 group vs. the CLP+O2 group. The lung histology score was lower for the SHAM group vs. the other groups. The Evans blue dye result was lower for the CLP+O3 group vs. the CLP+O2 group and the cecal ligation/puncture group but similar to that of the SHAM group. The survival rate for the CLP+O3 group was lower than for the SHAM group and similar to that for the other 2 groups (CLP and CLP+O2).
CONCLUSIONOzone therapy modulated the inflammatory response and acute lung injury in the cecal ligation/puncture infection model in rats, although there was no improvement on survival rates.
Despite the latest advances in antimicrobial therapy and intensive care, peritonitis and sepsis remain important causes of mortality. Infectious peritonitis can be due to intestinal perforation resulting from various conditions, including trauma, inflammatory diseases, ischemia and perforated neoplasms.1 Local infection triggers a massive release of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-10, platelet activating factor (PAF), leukotrienes, and thromboxane, all of which participate in the development of sepsis syndrome and septic shock.2,3
After initiation of the infection process, regardless of the optimal treatment, the inflammatory cascade is sometimes irreversible and results in patient death. Extensive research has focused on elucidating the mechanisms of the inflammatory cascade and modulation of these responses to prevent and treat Systemic Inflammatory Response Syndrome (SIRS) and Multiple Organ Dysfunction Syndrome (MODS)4,5.
Ozone (O3) is a potent oxidizing agent and an important disinfectant. The bactericidal effect of O3 is based on a direct attack on microorganisms by oxidation of biological materials. In fact, O3 antibacterial activity can be more effective than iodine and chlorine.6,7 Data reported in the literature have shown that bacteria, spores, and viruses are inactivated by ozone after only few minutes of exposure8,9. O3 may act through mechanisms other than oxidation, including the activation of erythrocyte metabolism and immune cells.10,11
Toxic effects of O3, caused by excessive doses of inhaled ozone in the airways, as well as toxicity to the endocrine, reproductive, and central nervous systems have been described7,12. Nevertheless, little is known about the intraperitoneal (i.p.) application of ozone or the repercussions that ozone has on intra-abdominal organs and tissues.
Two studies13,14 tested a preconditioning O2 /O3 pneumoperitoneum in rats submitted to peritonitis. The former study13 added an antibiotic regimen to the preconditioning pneumoperitoneum, and both found an increased survival rate in rats after the treatments. However, another study15 that evaluated preconditioning O2/O3 pneumoperitoneum demonstrated a pro-inflammatory effect of O3, with a tendency to reduce survival rates.
In this context, beyond these controversial results, the real effects of ozone therapy as a single therapeutic agent, not as a preconditioning treatment or as an adjuvant to antibiotics, have not been clearly determined. Thus, the objective of this study was to evaluate the effect of i.p. O3 application in a cecum ligation/puncture (CLP) rat model on the markers of the inflammatory response, on acute lung injury (ALI) parameters (pulmonary vascular permeability and histology), and on an animal’s survival rate.
MATERIALS AND METHODSThis study was performed in the Laboratory of Medical Investigation (LIM)-62 of the third Division of Surgical Clinic of the Hospital das Clinicas and the LIM 11 - University of São Paulo Medical School. Wistar male rats (250–350 g) were utilized throughout the experiments. This study was approved by our Institutional Ethics Committee and performed according to the National Institutes of Health (NIH) guidelines on the experimental use of animals.16 Four groups of rats were employed in this study:
- 1)
SHAM group animals were submitted only to laparotomy (hereafter this group is referred to as “group SHAM”);
- 2)
CLP group animals were submitted to laparotomy + CLP procedures (hereafter this group is referred to as “group CLP”);
- 3)
CLP+O2 group animals were submitted to laparotomy + CLP + O2 treatment (hereafter this group is referred to as “group CLP+O2”);
- 4)
CLP+O3 group animals were submitted to laparotomy + CLP + O3/O2 treatment (hereafter this group is referred to as “group CLP+O3”).
The lengths of the observation periods varied and are specified in the respective descriptive topics in this Methods section. The study design and distribution of the animals are shown in Figure 1. All of the animals fasted for 8 h before the surgical procedure. Laparotomy and cecal ligation/ puncture were performed under general anesthesia with inhalatory halothane, followed by i.p. pentobarbital injection (50 mg/kg).17–19
Study design. The animals were assigned to 4 groups: 1) SHAM, animals submitted to laparotomy and exposure of the cecum; 2) CLP, animals underwent cecal ligation/puncture and were observed; 3) CLP+O2, animals submitted to cecal ligation/puncture and were treated i.p. with O2; and 4) CLP+O3, animals submitted to cecal ligation/puncture and were treated i.p. with O3. For the survival analysis, n=40 (SHAM=10, CLP=12, CLP+O2=14, CLP+O3=14). Pulmonary vascular permeability was evaluated by the EBD lung leakage method in 40 animals (all groups with n=10). Cytokine sampling and histological analysis were studied in 44 animals (SHAM=10, CLP=11, CLP+O2=13, CLP+O3=10).
During the procedures, the animals were randomized to the 4 groups immediately after cecum exposure was accomplished. The SHAM rats were submitted to a 2-cm laparotomy, followed by exposure of the cecum and closure of the abdominal wall incision with a 4.0 nylon 2-layer running suture.
Cecum ligation and puncture and treatment with O2 or O2/O3After a 2-cm laparotomy on groups CLP, CLP+O2, and CLP+O3, the cecum was exposed, ligated with a 3.0 silk suture just distal to the ileocecal valve to avoid intestinal obstruction, and punctured once with a 22 g needle in its antimesenteric border. The cecum was then squeezed to expel a small amount of fecal material through the puncture site. The bowel was returned to the abdominal cavity, and the abdominal wall incision was closed, as described for group SHAM. The animals were kept warm (37º C) in the postoperative period. Food and water (with dipyrone 0.5%) were offered ad libitum after this procedure.18,20 For the treatment with O2 or O2/O3, the animals were anesthetized with halothane before the injection procedure. On group CLP+O2, 100% O2 (20 ml/kg) was insufflated (i.p.) every 12 h through a puncture in the right lower abdomen with a 22 gauge needle, starting 1 hour after the laparotomy closure. Similarly, group CLP+O3 received a gas mixture (20 ml/kg, i.p.) containing 5% ozone (102 μg/mL) and 95% oxygen, generated by a MVM™ ozone generator (Multivácuo®, Campinas, Brazil), every 12 h, starting 1 hour after laparotomy closure. After recovery from anesthesia recovery, the animals were allowed to have food and water (with dipyrone 0.5%) ad libitum during the observation period.
Enzyme-immuno assays for cytokines and chemokineSerum levels of the markers of the inflammatory response as well as the pulmonary histology changes were simultaneously evaluated in 44 animals (SHAM=10, CLP=11, CLP+O2=13, CLP+O3=10).
The serum concentrations of cytokines and chemokines (IL-6, IL-10 and CINC-1) were determined by enzyme-immuno assay (ELISA) using commercially available kits, according to the manufacturer’s instructions (R & D Systems Inc., Minneapolis, MN, USA). The sensitivity of the assay was 15 pg/mL.21
Pulmonary HistologyTwenty-four h after the initial procedure (laparotomy for group SHAM or CLP for the other groups), all of the animals were anesthetized with an injection of sodium pentobarbital (50 mg/kg, i.p.). A median sternotomy provided access to the thoracic cavity, and 5 ml of blood was collected from an intracardiac punction (22 gauge needle). This sample was used for cytokines and chemokine analysis. Euthanasia was then performed by cutting the left ventricle with scissors. A piece of the lower lobe of the left lung was fixed in 4% formaldehydum polymerisatum. Paraffin-embedded sections (5 μm) were stained with hematoxylin-eosin and submitted to morphological analysis, including extension (EX) of pulmonary involvement, edema (ED), hemorrhage (H), congestion (C), polymorphonuclear (PO) cell infiltrate and mononuclear (MO) cell infiltrate. A semi-quantitative analysis was performed, due to heterogeneous morphological changes and pulmonary involvement. A score (S) was calculated that combined the extension of pulmonary involvement and intensity of each morphological change, resulting in a final score [S= EX x (ED+H+C+PO+MO)]22. Two different pathologists conducted a blind histological analysis.
Pulmonary vascular permeability evaluationAcute lung injury was determined in 40 animals (SHAM=10, CLP=10, CLP+O2=10, CLP+O3=10) by evaluating the lung vascular permeability with the Evans blue dye (EBD) lung leakage method. Twenty-four h after the initial procedure (laparotomy for group SHAM or CLP in the other groups), the animals were anesthetized with an injection of sodium pentobarbital (50 mg/kg, i.p.), followed by the injection of 20 mg/kg EBD (delivered at a concentration of 25 mg/ml EBD in water, intravenous- (i.v.).
Fifteen minutes after the initial procedure, the laparotomy incision was reopened in all of the animals. Euthanasia was conducted by cutting the inferior vena cava. The thoracic cavity was then opened, and the lungs were perfused for 3 min with 50 ml phosphate-buffered saline (PBS) (pH 7.4) injected through a cannula inserted into the pulmonary artery. After dissection and en block removal of the heart and lungs, the left lung lobe was divided into two samples that were weighed to obtain the wet weight (WW) of each specimen. One sample was held in an oven at 100°C for 24 h and then weighed to determine its dry weight (DW). The dry to wet weight ratio (% DW/WW) of the lung tissue was then calculated. The other sample was submersed in formamide (4 ml formamide/g wet tissue) for 24 h to extract the dye from the tissue. The dye absorbance value in the solution was then detected by a microplate reader (wave length, 620 nm), compared with a series of standard EBD dilutions in formamide, and converted to μg of EBD/mL of the solution. Based on this value, the amount of EBD that had been in the wet lung sample (μg EBD/g wet tissue) was then determined. Finally, the %DW/WW was used to convert the wet tissue value into a μg EBD/g dry lung tissue value, which was recorded as the EBD lung leakage value.23,24
Survival analysisFor survival rate determination, the animals were observed until the 5th day post procedure. The survival rates were analyzed in 50 animals (SHAM=10, CLP=12, CLP+O2=14, CLP+O3=14), and all of the groups were observed for 5 days. The period of time from the initial procedure until death was annotated in h, and a Log-rank test was performed.
Statistical MethodsThe Kolmogorov-Smirnov test was used to determine normality. The data are presented as the mean ± SEM (when a normal distribution was found), or as the median (Md) plus the 25th and 75th percentiles [Md (P25;P75)] when the normality test failed. The parametric data were analyzed by ANOVA followed by the Bonferroni multiple comparisons test, when appropriate. The Kruskal-Wallis test was used to analyze the non-parametric data followed by the Student-Newman-Keuls test, when necessary. A Log-rank test was used for survival analysis. A p value < 0.05 was considered to be significant. Statistical analyses were conducted with Sigmastat® version 3.5 (Systat Software, Inc., Point Richmond, CA, USA).
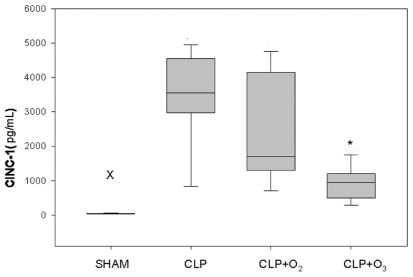
RESULTSCytokines and chemokine analysisThe levels of CINC-1 and IL-10 were expressed as medians (P25; P75), and the level of IL-6 was expressed as the mean ± SEM. CINC-1 levels (pg/mL) were significantly lower for group CLP+O3 [951.3 (582.6; 1111.5)] than for group CLP+O2 [1700 (1300; 3928.1)] and group CLP [3555 (3066.8; 4404)]. Group SHAM had the lowest CINC-1 values [47 (43.2; 53.8)] (p<0.01). The Kruskal-Wallis test, followed by the Student-Newman-Keuls test, found no significant difference between groups CLP and CLP+O2 (Figure 2).
Kruskal-Wallis test for CINC-1 levels 24 h after the initial procedure. SHAM animals had the lowest levels of all groups. Group CLP+O3 had CINC-1 levels significantly lower than groups CLP and CLP+O2.X Group SHAM vs. other groups (P<0.01). * Group CLP+O3vs. groups CLP and CLP+O2 (P<0.05). Values of CINC-1 are medians (P25;P75) expressed in pg/mL.
IL-6 levels (pg/mL) were significantly lower for group SHAM (30.8 ± 4.8) than for all the other groups (p<0.001). Groups CLP+O3 (321.3 ± 35.2) and CLP+O2 (386.3 ± 40.9) had lower levels than group CLP (695 ± 65.3) (p<0.01). A comparison between groups CLP+O3 and CLP+O2 showed no significant difference (p=0.54).
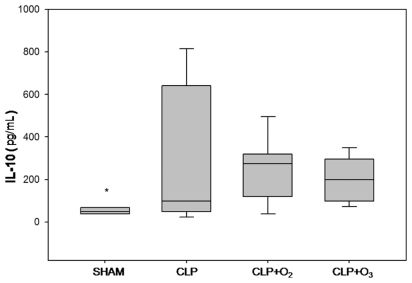
IL-10 levels (pg/mL) for the four groups were 50.1 (38.8; 67) for group SHAM, 98.1 (56.1; 598) for group CLP, 273.6 (152; 314.8) for group CLP+O2, and 199.5 (107; 289) for group CLP+O3. The Kruskal-Wallis test showed that IL-10 levels were lower for group SHAM (p=0.02) than for the other groups. There was no difference between the other 3 groups (p=0.85) (Figure 3).
Pulmonary HistologyFor lung histology and cytokines analysis, we used 10 rats for group SHAM and, initially, 13 animals in each CLP procedure group. Two animals of group CLP and 3 animals of the group CLP+O3 had an inadequate blood sample (hemolyzed plasma), so we eliminated these animals. SHAM rats had the lowest histology score [3(2; 4)] of all groups (p=0.002). The pulmonary histology scores were similar in groups CLP+O2 [6 (3.7; 10.5)], CLP+O3 [8 (6; 8)], and CLP [10 (5; 12)]. The Kruskal-Wallis test showed no differences between the three groups (p=0.31).
EBD lung leakage methodThe EBD lung leakage values were 62.5 (39; 71.3) for group SHAM, 56.1 (52.7; 109.1) for group CLP, 88.2 (53.6; 151.1) for group CLP+O2, and 28.4 (18.4; 51.3) for group CLP+O3. There was a significant difference when we compared group CLP+O3 with groups CLP+O2 and CLP (p=0.02). A comparison of groups CLP+O3 and SHAM showed no significant difference.
Survival AnalysisFor the survival analysis, we used 10 animals for group SHAM because of the low morbidity of the procedure. The groups with the CLP procedures initially had 14 animals, but 2 animals of group CLP died right after anesthesia and then were eliminated from the analysis.
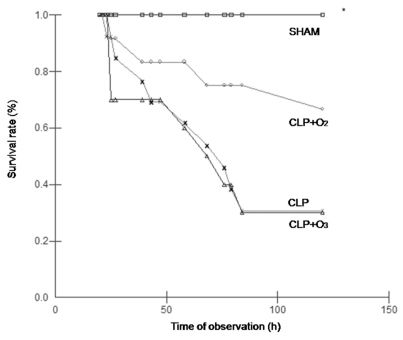
Only one animal of group SHAM died during the observation period (110 ± 10 h). All of the other groups had a decreased survival time compared to group SHAM (p=0.002). The observed death rates were: 1/10 (10%) for group SHAM, 9/12 (75%) for group CLP, 6/14 (42.8%) for group CLP+ O2, and 10/14 (71.4%) for group CLP+O3. Animals of group CLP+O2 had a survival time that was not significantly different from that of group CLP+O3 (71 h ± 12.9 vs. 52.1 ± 8 h, p=0.4). Animals of group CLP had a mean survival time of 57.4 ± 10.3 h. The Log-rank test performed between groups CLP, CLP+O2, and CLP+O3 showed that there was no statistical difference (p=0.4). These results are illustrated in Figure 4.
Log – rank test. The observation period was not completed on only one animal from group SHAM. All of the other groups had a decreased survival time compared to group SHAM (p=0.002). There were no differences in survival among groups CLP, CLP+O2, and CLP+O3 (p>0.05). □ = SHAM; ▵ =CLP; ○ = CLP+O2; × = CLP+O3.
The utilization of O3 as a potent antimicrobial agent has been the object of many studies.25–30 The use of ozone in humans and animals is still considered controversial because of its side effects that are specifically related to free radical formation and irritation of the respiratory system.12, 31 For these reasons, ozone therapy has had limited acceptance in clinical practice, and most of the studies on O3 therapy have been experimental. Despite this fact, ozonized oxygen exhibits various effects on the immune system, such as the modulation of phagocytic activity on the peritoneal32 and alveolar33 macrophages that generate the first line of defense against bacteria and/or its toxins.
Bette et al. (2006)13 tested a preconditioning O2/O3 pneumoperitoneum, followed by a tazobactam/piperacilin regimen in Wistar rats submitted to peritonitis, and found an increase in survival rates and a decrease in pro-inflammatory cytokines (TNF-α and IL-1β). Promisingly, Schulz et al. (2003)14 found that after a repetitive pre-treatment using ozonized oxygen (i.p.), mortality in animals submitted to lethal polymicrobial peritonitis was reduced. Rodriguez et al. (2009)1 verified that there was an increase of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in rats submitted to ozone oxidative preconditioning (OOP) prior to a fecal peritonitis induction, suggesting that there was a protective effect of OOP. However, in a model of peritoneal sepsis in rats submitted to a 5-day preconditioning period with O3 (i.p), Torossian et al. (2004)15 found that survival was reduced from 50% to 35% (p=0.1). In addition, ozone therapy increased TNF-α and macrophageal inflammatory protein (MIP)-2. From this, the authors concluded that O3 therapy (i.p.) was proinflammatory 15.
The previous studies with O3 preconditioning1,14,15 or preconditioning plus antibiotics13 may not have correctly estimated the true impact of ozone therapy in the treatment of septic rats. Therefore, we conducted this study to evaluate the isolated effect of intraperitoneal ozone as a single therapeutic agent in intra-abdominal infections.
In this study, the variables were chosen based on the previous reports in the literature1,7, 13,14,15,20 and for their applicability for the evaluation of SIRS.1,21,24
The CLP procedure groups were substantially affected for CINC-1 elevation compared to group SHAM. Group CLP+O3 had a significant decrease in CINC-1 levels when compared to all of the CLP-procedure groups. CINC-1 plays an important role in the inflammatory response as an attractant and activator of neutrophils and macrophages.34,35 However, a previous in vitro study36 demonstrated that adhesion and chemotaxis of neutrophils were independent of exposure to ozone when peripheral blood was used. CINC-1 levels rapidly increase after induction of inflammation in rats, especially 8 hrs after the injury35, and the levels reach concentrations significantly different from those of controls at 4, 8, and 24 hrs after LPS infusion in blood samples.37 The possible mechanism by which CINC-1 expression was modulated in this study remains to be determined. We hypothesize that inactivation of bacteria, as a result of topical (i.p.) application of ozone, could attenuate the migration of macrophages, decreasing CINC-1 expression at 24 h. A histological analysis of the peritoneum after euthanasia by counting the macrophages could provide additional evidence for this hypothesiIL-6 is secreted from T-cells and macrophages and stimulates the immune response to trauma, leading to inflammation. In mice, IL-6 has been shown to be required for resistance against certain species of bacteria, acting as a weak pyrogen.38 The CLP procedures increased IL-6 values compared to group SHAM, but groups CLP+O3 and CLP+O2 had lower levels of IL-6 than group CLP, and no difference was observed when group CLP+O3 was compared to group CLP+O2. Inactivation of bacteria by ozone could explain this decrease in IL-6 levels for group CLP+O3, but there is no clear mechanism to explain the similar expression of IL-6 for group CLP+O2 compared to CLP+O3.
Intraperitoneal administration of O2 and O3 did not have a significant impact on IL-10 levels after 24 h of observation. An IL-10 peak can be observed as much as 24 h after systemic injury.39 Anti-inflammatory IL-10 production is known to be part of a protective mechanism that suppresses the induction of proinflammatory cytokines such as TNF-α and IL-1 and is itself induced by monocytes and macrophages during sepsis.40, 41 Sewnath et al. (2001)42 found a role of endogenous IL-10 in local antibacterial host defense and in the development of SIRS during abdominal sepsis, indicating that endogenous IL-10 increases bacterial clearance.
Previously, it has been shown that an increased histology score is associated with the presence of ALI.43 In the CLP groups, the increased histology scores indicate that the CLP procedures provoked pulmonary tissue injury. However, there was no beneficial effect of O3 therapy on the lung histology score. Hyaline membranes were not found in any of the animals, and this compromised the characterization of ALI by histological methods in this study. This might be attributed to the short time interval (24 h) between the CLP procedures and euthanasia of the animals. Hyaline membranes can be identified by optical microscopy 24 to 48 h after initial injury.43 The presence of edema in the samples that were evaluated 24 h post-injury was uniformly discreet, and thus we decided to eliminate it in calculating the histology score. The ALI analysis might have been more accurate if pulmonary histology had been performed 48 h later.
The EBD lung leakage method was chosen because it is an early marker of lung vascular injury.23,24 Differences in pulmonary vascular permeability can be observed as soon as 2 h after procedures such as gut ischemia/reperfusion and lipopolysaccharide (LPS) infusion.23,24 Moreover, accumulation of macrophages at sites of tissue injury can be observed after acute inhalation of ozone.44,45 These cells, along with resident alveolar epithelial cells, become activated and release cytotoxic and proinflammatory mediators, such as nitric oxide (NO), which increase of pulmonary vascular permeability and to the development of ALI.44,45 However, in the present study (with i.p. administration of O3), there was a less pronounced capillary permeability (quantified by means of EBD lung leakage) for group CLP+O3, and this might suggest that O3 therapy had an anti-inflammatory effect. The administration route (i.p.) might have better controlled the intraperitoneal infection7 without the undesirable side effects of ozone inhalation. The modulation of the inflammatory cascade might explain the lower EBD lung leakage values for group CLP+O3vs. groups CLP+O2 and CLP. There was no difference between groups CLP+O3 and SHAM. This might be due to the small influence that laparotomy and cecal exposure have on pulmonary vascular permeability for group SHAM.
We evaluated the survival rate 5 days after procedure, quantified the survival time in h for each animal, and performed a Log-rank test. We used a concentration of 102 μg/ml O3 with a lower volume of the O2/O3 gas mixture (20 ml/kg). We used a different concentration from that used by Schulz et al.14 (80 mL/kg) to avoid any potential side effects of massive pneumoperitoneum, but the final concentration of infused ozone was similar. The CLP groups had increased mortality compared to group SHAM (p=0.002), but no difference was observed when each group was compared to the other (p=0.12). We observed that group CLP had a similar lethality rate (84%) compared to the classic study by Wichterman et al. (1980)20 on survival rates after CLP. Thus, in the present study, ozone treatment did not influence survival rates.
In conclusion, the results of our study suggest that intraperitoneal ozone therapy has a possible benefit in attenuating the inflammatory response and acute lung injury resulting from intra-abdominal infection in rats, although there was no improvement in survival rates.