To evaluate the histological changes of tracheal cartilage and epithelium caused by tracheal occlusion at different gestational ages in a fetal rat model.
METHODS:Rat fetuses were divided into two groups: a) External control, composed of non-operated rats, and b) Interventional group, composed of rats operated upon on gestational day 18.5 (term = 22 days), divided into triads: 1) Tracheal occlusion, 2) Internal control and 3) Sham (manipulated but not operated). Morphological data for body weight, total lung weight and total lung weight/body weight ratio were collected and measured on gestational days 19.5, 20.5 and 21.5. Tracheal samples were histologically processed, and epithelial, chondral and total tracheal thicknesses were measured on each gestational day.
RESULTS:The tracheal occlusion group exhibited an increase in total lung weight/body weight ratio (p<0.001). Histologically, this group had a thicker epithelial thickness (p<0.05) and thinner chondral (p<0.05) and total tracheal thicknesses (p<0.001). These differences were more prominent on gestational days 20.5 and 21.5.
CONCLUSION:Tracheal occlusion changed tracheal morphology, increased epithelial thickness and considerably decreased total tracheal thickness. These changes in the tracheal wall could explain the development of tracheomegaly, recently reported in some human fetuses subjected to tracheal occlusion.
Congenital diaphragmatic hernia (CDH) is an embryological defect of the diaphragm that occurs in approximately 1:2500 live births and accounts for 8% of the major congenital anomalies. It occurs because of a defect in the development of the pleuroperitoneal folds, leading to the passage of abdominal organs into the thorax. The presence of abdominal viscera in the thorax, usually the intestine and liver, occupies space, thus compressing the lung, impairing its normal development, and causing a defect in lung maturation and consequent pulmonary hypoplasia and hypertension (1,2).
Fetal tracheal occlusion (TO) via FETO (Fetoscopic Endoluminal Tracheal Occlusion) aims to promote in utero lung growth and decrease neonatal CDH mortality (3,4). TO prevents the amniotic fluid outflow from the lungs into the amniotic cavity; through mechanical action, it causes accelerated pulmonary growth, alveolarization and alveolar distension, decreasing the deleterious effects of pulmonary hypoplasia and hypertension (5-7). After TO removal, there is a large increase in the amount of intrapulmonary mucus. It is unclear whether this is due to the accumulation of mucus as a result of TO or the increased production of mucus by goblet cells in the tracheal epithelium (6).
Yoshizawa et al. (8) concluded that TO stimulates cell cycle progression and type I lung cell differentiation in rat fetuses. However, TO has adverse effects on type II cells and induces a surfactant deficiency, which depends on pulmonary maturity and occlusion duration. It is possible that the effects of TO, both adverse and beneficial, are not only restricted to the lungs but may also extend to the trachea. It is known that the balloon causes slight changes in the trachea, such as local inflammatory changes and limited epithelial defects, such as a reduction in air contact surface (9). However, these changes are not well understood. Thus, using the experimental model of TO, we assessed the histological changes caused by the mechanical effects of TO on the trachea at different gestational ages.
METHODSAnimalsThis study was approved by the Ethics Committee on Animal Research of the School of Medicine of Ribeirão Preto of the University of São Paulo – USP – (#043/2011) and followed the Council for International Organization of Medical Sciences (CIOMS) ethical code for animal experimentation and the principles of the Brazilian College on Animal Experimentation. Sprague-Dawley rats were kept under a controlled dark-night cycle with ad libitum food and water supply. The animals were mated during the night cycle, and a sperm-positive vaginal smear on the following day confirmed mating and designated gestational day (GD) 0 (term = 22 days).
Experimental groupsPregnant rats were divided into two groups: external control (EC, n = 6) and interventional group (IT, n = 15). EC rats were not subjected to any treatment. IT rats were subjected to surgery on GD 18.5, forming triads: internal control (C), sham (S) and TO.
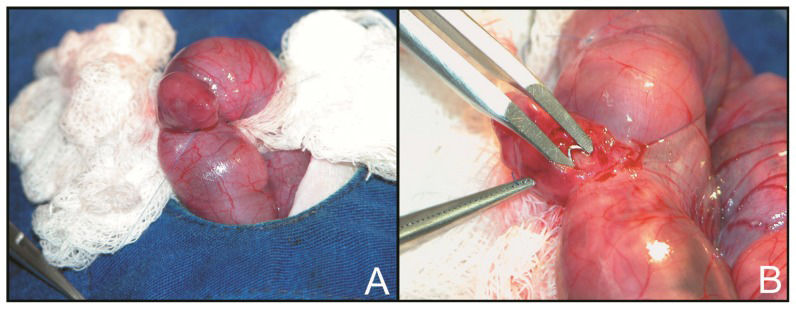
Surgical procedure for tracheal occlusionOn GD 18.5, time-pregnant rats were anesthetized, and a median laparotomy was performed under sterile conditions. The uterine horn was exposed, and a purse string suture was placed in the uterine wall near the fetal head. A hysterotomy was performed, and the fetal head and neck were exposed. The neck was incised, and the trachea was isolated and occluded on its upper portion using a titanium microclip (Teleflex Medical Research Triangle Park, NC, USA) (Figure 1). The incision was not sutured. The fetus was then returned to the amniotic cavity, the amniotic fluid was replaced with saline 0.9% and the purse string was tightened. Sham fetuses were subjected to a similar procedure without cervical incision and tracheal occlusion; internal control fetuses were not manipulated. This sequence (TO, C and S) was repeated as many times as possible in each uterine horn. The uterine horn was then returned to the peritoneal cavity, and the abdominal wall was closed in two layers.
Sample harvestingEach of the four groups was harvested on GD 19.5, 20.5 and 21.5, forming a total of twelve sub-groups. Each sub-group was composed of 12 fetuses, with a total of 144 fetuses. On harvest day, the rats were anesthetized and subjected to Caesarian section. Fetuses were removed from the uterus and weighed. After weighing, the fetuses were sacrificed by occipital puncture, the thorax and abdomen were opened, and the lungs and trachea were carefully removed and weighed. A proper TO was confirmed by observation of distended lungs. Tracheas were dissected under a 2.5× magnification loupe, and for TO fetuses, tracheal samples were cut one or two rings below the clip area. Samples were fixed in formaldehyde 10%.
Morphometrical evaluationBody weight (BW) and total lung weight (TLW) allowed for a statistical analysis of these variables in all groups. A TLW/BW ratio was calculated to exclude the variable BW from the TLW evaluation. Twelve fetuses were weighed in each sub-group.
Histological evaluationSamples were cut into 5-μm axial sections, starting below the cricoid cartilage and progressing towards the carina. Sections were stained with H/E for measurements and cell count evaluation. Histological sections were photographed under 40× magnification, and digital images were analyzed using Image Pro Plus 6.0 software for measurement of epithelial thickness (ET), chondral thickness (CT) and total tracheal thickness (TTT) in μm and epithelial cell count (ECC). Each section was divided into four quadrants, using six sections for each trachea. A mean of measurements for the four quadrants was used because of the irregular form of the trachea. The pars membranacea was not studied because it is too thin in rat fetuses. Using six slices per trachea, epithelial cells were manually counted in the whole slice, with each nucleus corresponding to a cell. Six fetuses were studied in each sub-group.
Statistical analysisMorphological and histological variables were described as the means ± standard deviations (SD) and analyzed using the ANOVA method followed by the Tukey-Kramer post-test in GraphPad Prism 3.02. A difference with a p<0.05 was considered significant.
RESULTSMorphometrical resultsAll fetuses subjected to surgery on GD 19.5 had a lower initial BW compared with EC fetuses (p<0.001) (Figure 2-A). TLW in TO fetuses progressively increased, differing from the normal curve observed in the EC, C and S groups (Figure 2-B). The TLW/BW ratio indicated the magnitude of this weight gain (Figure 2-C). This increase was so significant that on GD 21.5, the weight loss due to surgical intervention was reversed, and the BW of TO fetuses was similar to EC fetuses (p>0.05). Morphometrical results and differences are shown in Table 1.
Morphometrical results in the different groups, expressed as the means and SD.
| GD | EC | C | S | TO | p-value | |
|---|---|---|---|---|---|---|
| BW (g) | 19.5 | 4.027 (±0.301) | 2.693 (±0.169) | 2.617 (±0.170) | 2.793 (±0.245) | p<0.001 A,B,C |
| 20.5 | 5.403 (±0.401) | 4.017 (±0.286) | 3.996 (±0.380) | 4.001 (±0.477) | p<0.001 A,B,C | |
| 21.5 | 5.769 (±0.322) | 4.881 (±0.444) | 4.908 (±0.163) | 5.630 (±0.799) | p<0.05 E,F p<0.001 A,B | |
| TLW (g) | 19.5 | 0.134 (±0.013) | 0.076 (±0.009) | 0.068 (±0.019) | 0.091 (±0.024) | p<0.001 A,B,C,F |
| 20.5 | 0.137 (±0.012) | 0.111 (±0.021) | 0.109 (±0.021) | 0.239 (±0.084) | p<0.001 C,E,F | |
| 21.5 | 0.177 (±0.016) | 0.119 (±0.011) | 0.115 (±0.010) | 0.422 (±0.123) | p<0.001 C,E,F | |
| TLW/BW (g) | 19.5 | 0.033 (±0.0020) | 0.028 (±0.0039) | 0.026 (±0.0100) | 0.033 (±0.0100) | NS |
| 20.5 | 0.025 (±0.0016) | 0.028 (±0.0010) | 0.027 (±0.0043) | 0.060 (±0.0200) | p<0.001 C,E,F | |
| 21.5 | 0.031 (±0.0020) | 0.024 (±0.0021) | 0.023 (±0.0017) | 0.075 (±0.0100) | p<0.05 A,B p<0.001 C,E,F |
GD: Gestational day; EC: External control; C: Control; S: Sham; TO: Tracheal occlusion; BW: Body weight; TLW: Total lung weight; TLW/BW: TLW/BW ratio. NS: Non-significant, A: EC x C; B: EC x S; C: EC x TO; D: C x S; E: C x TO; F: S x TO.
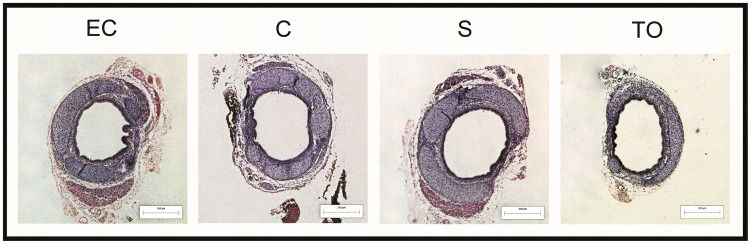
Despite the lower BW of operated fetuses, an initial increased ET was observed compared with EC fetuses, but the difference was not statistically significant. This difference was reversed in the following harvests, except for TO fetuses, where a thicker epithelium was observed on GD 21.5 compared with C and S fetuses (p<0.05) (Figure 3-A). It was also observed that the tracheal cartilage of the TO group was progressively thinner compared with EC (GD 20.5 and GD 21.5 = p<0.05) and C fetuses (GD 21.5 = p<0.05) (Figure 3-B). In general, it was observed that tracheal cartilage was responsible for 60-70% of TTT in this study. Additionally, similar results were noted in the CT and TTT analysis. TO fetuses had a thinner TTT compared with EC (p<0.001), C (p<0.05) and S fetuses (p<0.05) on GD 21.5 (Figure 3-C). TO led to an increase in ECC after the first post-operative day compared with other groups (p<0.001) (Figure 3-D). Histological sections from all groups are shown in Figure 4. Histological results and differences are shown in Table 2.
Histological results in the different groups, expressed as the means and SD.
| GD | EC | C | S | TO | p-value | |
|---|---|---|---|---|---|---|
| ET (μm) | 19.5 | 16.17 (±2.427) | 16.88 (±2.285) | 16.73 (±2.848) | 17.01 (±1.703) | NS |
| 20.5 | 14.00 (±1.638) | 14.76 (±1.977) | 14.41 (±1.518) | 14.00 (±1.470) | NS | |
| 21.5 | 12.95 (±1.901) | 11.57 (±1.638) | 11.41 (±1.128) | 14.45 (±1.378) | p<0.05 E,F | |
| CT (μm) | 19.5 | 80.32 (±13.982) | 71.49 (±11.501) | 68.86 (±7.045) | 67.35 (±11.780) | NS |
| 20.5 | 98.13 (±6.322) | 94.55 (±8.420) | 86.95 (±13.262) | 78.12 (±13.014) | p<0.05 C | |
| 21.5 | 99.40 (±11.104) | 96.47 (±13.773) | 90.40 (±9.826) | 72.19 (±12.024) | p<0.05 C,E | |
| TTT (μm) | 19.5 | 147.93 (±32.475) | 118.40 (±16.960) | 113.44 (±9.598) | 111.56 (±10.138) | p<0.05 B,C |
| 20.5 | 138.62 (±12.302) | 142.44 (±7.142) | 132.68 (±14.606) | 119.12 (±13.084) | p<0.05 E | |
| 21.5 | 140.62 (±10.906) | 136.67 (±15.416) | 127.61 (±14.223) | 102.12 (±12.965) | p<0.05 E,F, p<0.001 C | |
| ECC (cell number) | 19.5 | 287 (±33.187) | 311 (±14.980) | 312 (±11.165) | 382 (±12.215) | p<0.001 C,E,F |
| 20.5 | 291 (±20.928) | 314 (±11.296) | 318 (±7.521) | 398 (±16.346) | p<0.05 B, p<0.001 C,E,F | |
| 21.5 | 295 (±13.277) | 323 (±24.367) | 325 (±9.704) | 401 (±48.798) | p<0.001 C,E,F |
GD: Gestational day; EC: External control; C: Control; S: Sham; TO: Tracheal occlusion; ET: Epithelial thickness; CT: Chondral thickness; TTT: Total tracheal thickness; ECC: Epithelial cell count. NS: Non-significant, A: EC x C; B: EC x S; C: EC x TO; D: C x S; E: C x TO; F: S x TO.
Temporary TO fosters clinical and experimental growth of hypoplastic lungs in CDH (4,5); the experimental rat model is effective for the study of lung and tracheal development (10). The TLW/BW ratio demonstrated that TO was effective and validated the mechanical action on the trachea, revealing the most significant increase between GD 20.5 and 21.5. TO acted as a barrier to fluid outflow produced by the lungs, leading to expansion and weight gain; similar results were observed by Kitano et al. (11).
Except for the TO group, tracheal epithelium thickness was the thickest on GD 19.5, decreased during the course of pregnancy, and was considerably thinner at term. In the TO group, the epithelial thickness decreased between days 19.5 and 20.5 and increased on GD 21.5, which was different than the other groups (p<0.001). Similar results were found for ECC, where an increase was observed in the TO group compared with the other groups (p<0.001). This increase in ET and ECC could explain the increased mucus production reported by Harrison et al. (6).
Tracheal cartilage thickness increased in groups EC, C and Sham over time, unlike the TO group, in which the cartilage initially increased after the placement of the clip and decreased significantly at the end of gestation (p<0.001). Thus, it can be inferred that the TTT reduction in the TO group occurs due to a reduction in cartilage and other components of the tracheal ring, such as the lamina propria and the submucosal layer; this reduction is even more important considering that the epithelium was thicker.
This could be explained by mechanical stimulation from the accumulation in the airways of the fluid produced by the lung, which acted on the tracheal epithelium, but not over the whole trachea, because there was a reduction in TTT, with a thinner cartilage. Although there is a difference between the TO procedure in rats and humans, which is performed externally using a clip in this experimental model and performed by FETO using an intra-tracheal balloon in humans, our results support the idea that, as suggested by Yoshizawa et al. (8), both pulmonary and tracheal epithelial growth could be aligned.
In premature animals, there is a decreased capacity of the cartilage rings and tracheal muscle to withstand pressure, leading to greater tracheal compliance (12,13). As described by Deoras et al., the acute distension of the trachea of extremely premature sheep neonates promotes a major distortion of the tracheal architecture, especially affecting the muscular posterior wall of the trachea (pars membranacea), leading to a decrease in tracheal cartilage thickness (14). Although the TO duration is short, these changes could be explained by Laplace's law (γ = P x r/h), where the constant pressure of the amniotic fluid would exert a pressure on the tracheal tube, in an attempt to decrease the wall’s thickness.
Deprest et al., when performing TO using a balloon or clip in a sheep model, did not observe changes in the tracheal cartilage thickness, which differed from our results. However, in that study, the impact of balloon placement on the tracheal wall and its recovery after the TO removal were evaluated, emphasizing the inflammatory aspect. In addition, in the evaluation of the area below TO, an epithelial unfolding was observed (9). We have not assessed the epithelial folding degree, but we noted a thickening of that layer. Apparently, in the rat model, no epithelial folding was observed in either the TO or the other groups. The explanation for this difference may be intrinsic to histological conformations of these animals.
Human FETO has increased survival in severe CDH cases (15,16). Recently, tracheomegaly was observed at later follow-up of children undergoing FETO (17,18). Given the limitations of our study that are related to the TO method and the use of a small animal, our results support the idea that after TO, there may be a thinning of the tracheal cartilage, which could explain tracheomegaly. The long-term follow-up of a larger number of patients subjected to FETO will tell us if these changes in tracheal diameter will persist. In addition, we observed epithelial thickening associated with increased cellularity, which could lead to increased mucus production. This difference in the amount of epithelial cells encourages further studies to distinguish which cell types are increased in the tracheal epithelium.
FAPESP – São Paulo Research Foundation – Research Grant #11/00794-1, Scholarships #08/52772-9 and #11/12587-0 and CAPES (Higher Education Consortia Program - Brazilian Ministry of Education).
No potential conflict of interest was reported.
Gallindo RM and Gonçalves FL contributed to the preparation of the manuscript and experimental surgery procedure. Barreto CT contributed to the animal care and experimental surgery procedure. Schmidt AF contributed to the animal care, statistical analysis and English correction. Pereira LA performed the analysis of histologic samples and prepared the manuscript. Sbragia L contributed to the experimental surgery procedure and prepared the manuscript.