The oral cavity is a link between of external environment with gastrointestinal tract. Studies are controversial on the presence of Periodontal Disease (PD) and its association with Gastric Adenocarcinoma (GAC).
MethodsThe authors performed a systematic review and meta-analysis to verify the association between PD and GAC. Six electronic databases were evaluated between 1961 and 2022. Titles and abstracts were reviewed independently according to the eligibility criteria, assessing full texts of selected studies. The quality of the included research was verified using the Newcastle-Ottawa Scale for case-control and cohort studies. Statistical analyses were performed based on fixed and/or random effects models to calculate the summarized Relative Risk (RR) and its 95 % Confidence Interval (95 % CI).
ResultsThere were 639 studies, of which nine articles were included (3 case-controls and 6 cohorts). Overall, the authors identified 1,253 cases of GAC 2,501 controls in case-control studies, and 1,631 patients with GAC enrolled in cohort studies. Patients presenting PD increased the risk of developing GAC by 17 % (RR=1.17; 95 % CI 1.03‒1.32), which remained regardless of the diagnostic method for PD, i.e., clinical examination (RR = 1.19; 95 % CI 1.14‒1.24) and self-report (RR = 1.34; 95 % CI 1.06‒1.69). Moreover, Asian patients (RR=1.17; 95 % CI 1.00‒1.36) with PD had a higher risk of having GAC than American and European patients (RR = 1.18; 95 % CI 0.84‒1.66).
ConclusionsThe presence of PD the risk of GAC suggesting that its infectious-inflammatory process of PD may be related to GAC development. Further investigations on the oral-gastric microbiota and its role in the carcinogenesis of gastric cancer should be carried out, and the screening of patients with potential risk for GAC should be considered in the clinical practice of dentists.
In 2020, Gastric Cancer (GC) was the malignancy that had more than one million new cases and about 770,000 deaths worldwide. Being it is fifth most frequent cancer and the fourth cause of cancer death in the world.1 Its risk factors already known, in addition to infection by Helicobacter pylori (H. pylori), are obesity, excessive intake of salt and meat, low consumption of fruits and vegetables, smoking, alcoholism, and low socioeconomic status, they are associated with gastric carcinoma and other malignancies.2,3
Periodontal diseases affect up to 50 % of the world's population and rank sixth among the most prevalent pathologies worldwide.4 Alterations in oral dysbioses change the oral microbiome, which may lead to oral pathologies such as Periodontal Disease (PD). Gingivitis and periodontitis are the most common forms of PD. This disease has different clinical signs of inflammation limited to the gum (gingivitis), while periodontitis results in progressive destruction of the periodontal ligament and alveolar bone, forming pouch, gingival retraction, or both5,6 and tooth loss is considered a result a significant increase in periodontal diseases in individuals over 40 years of age.7 Therefore, there is some evidence about that dysbiosis occurring in the oral cavity, such as periodontal disease, is a trigger for cancer, such as gastric adenocarcinoma.8,9
But epidemiological evidence on the association of PD and GAC remains limited and controversial, where some studies suggest positive associations, reporting that the infectious-inflammatory process of PD is capable of initiating inflammation mediators and microorganisms that may initiate the carcinogenesis10–15 however some studies had null results, mainly due to, lack of pattern about data on exposure.16–18 This study aimed to conduct a systematic review with meta-analysis to investigate the an association between PD and GAC.
Materials and methodsThis project was registered on the International Prospective Register of Systematic Reviews ‒ PROSPERO platform on January 18, 2021, under registration code CRD42021221317.
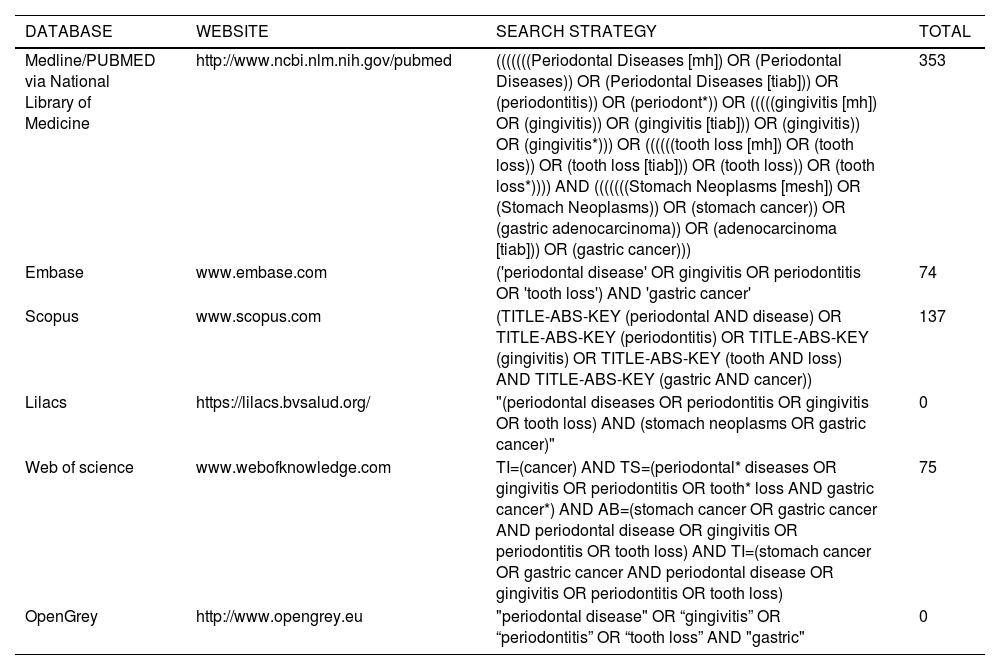
Literature searchThis systematic review was conducted and reported in accordance with PRISMA guidelines.19 Articles were identified through searches limited to the English language on the PubMed, Embase, Web of Science, Scopus, Lilacs and Opengrey databases. The search strategy was based on different terms for each database (Table 1).
Search strategy.
In this study, the presence of PD was considered whether, at least, one of these clinical characteristics occurs: gingivitis, periodontitis and tooth loss.5,20 This systematic review included case-controls and cohorts studies, and the authors excluded cross-sectional, experimental, animal studies, and case reports. Thus, the authors used the Rayyan software to identify eligible studies and exclude duplicates.21 The researchers (FJNA and MAF) retrieved data from studies independently based on titles and abstracts of the eligible studies according to the question of the systematic review (Are patients with periodontal disease at risk for developing gastric adenocarcinoma?). Moreover, references of the selected articles were reviewed to find relevant studies.
Data extractionThe following variables were collected: (1) First author, year of the publication, and place of the study; (2) Type and period of the study; (3) Sample size, sex, and age; (4) Exposure presence of PD; (5) Diagnosis method for the exposure; (6) Outcome gastric cancer; (7) Diagnosis methods: self-declare and clinical examination; (8) Association measures and 95 % CI (OR, RR, HR); and (9) Adjustment variables according to the articles reviewed: Sex; Smoking; Alcohol; socioeconomic status; Intake of vegetables and fruits; BMI; Regular physical activity among others (Tables 2 and 3). The discrepancies between the two reviewers were solved with the participation of a third evaluator (FSM).
Characteristics of the case-control studies included in the systematic review.
CI, confidence interval; F, female; M, male; CG, gastric cancer; OR, odds ratio; RR, relative risk; HR, hazard ratio; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICD, international classification of diseases.
Characteristics of cohort studies included in the systematic review.
CI, confidence interval; F, female; M, male; CG, gastric cancer; OR, odds ratio; RR, relative risk; HR, hazard ratio; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DHF, international classification of diseases; DMFT, decayes, missing and filled teeth.
The Newcastle-Ottawa Scale (NOS) was applied to evaluate the quality of selected studies, by two independent, previously trained and approved reviewers. The methodological is divided into three components: group selection (0‒4 points), quality of adjustment for confounding (0‒2 points), and exposure assessment after outcome (0‒3 points). The maximum score can be 9 points, which represents high methodological quality.22 A the funnel plot was carried out to assess the risk of publication bias.23
Statistical analysisFor this meta-analysis, the authors considered the following measures of association: Odds Ratio (OR); Relative Risk (RR): Hazard Ratio (HR) and their respective 95 % Confidence Intervals (95 % CI). Accordingly, the authors carried out fixed and random effects models using the “metan” command.24 The heterogeneity among the studies was assessed by the I2 statistic, where I2 = 0‒25 % indicated low heterogeneity; I2 = 25 %‒50 %, moderate heterogeneity; and I2 > 50 %, high heterogeneity.25 The authors used the random effects model in case of high heterogeneity among studies and the software to perform the meta-analysis was STATA 15.
ResultsStudy selectionThe authors have found 639 articles between 1961 and 2022 (Fig. 1). After reading their title/abstract, the study excluded 441. From that 40 articles were considered eligible for a full reading. Seven studies were shortlisted for the meta-analysis,16,26–31 two additional articles were included.32,33 Thus, nine articles were included in this systematic review, three case control studies26,27,32 and six cohort studies16,28–31,33 published between 1998 and 2018. The authors found a population of 2884 cases patients with GAC in the nine studies included.
About six studies were carried out in Asia accounting 2280 gastric adenocarcinoma cases and the diagnostic criteria for PD was the clinical examination (Tables 2 and 3).
In cohort studies, the NOS ranged from eight points30,33 to nine points,16,28,29,31 and between six and eight points26,27,32 among case-control studies selected therefore selected studies have high scores in quality (Fig. 2).
In cohort studies, the NOS ranged from eight points30,33 to nine points.
Summary of meta-analysisIn the meta-analysis of nine observational studies, the presence of PD was associated to an increase in the risk of GAC by 17 % (RR = 1.17; 95 % CI 1.03‒1.32), with a heterogeneity of 39.9 % (Fig. 3). While in the subgroup analysis, cohort and case-control studies had no association with PD and GAC (Fig. 4).
An association between GAC and PD was found to be a risk in the Asian population (RR = 1.17; 95 % CI 1.00‒1.36); however, in American and European studies there was no risk (RR = 1.18; 95 % CI 0.84‒1.66) (Fig. 5).
To evaluate the presence of PD In epidemiological studies, it can be assessed by patient self-report and by clinical examination. The authors observed an increased risk of patients with PD presenting GAC, that remained regardless of the diagnostic method for PD, 19 % (RR=1.19; 95 % CI 1.14‒1.24) to 34 % (RR = 1.34; 95 % CI 1.06‒1.69) for clinical examination and self-report, respectively (Fig. 6).
No publication bias was observed in the selected studies according to the Egger test (p = 0.860) (Fig. 7).
DiscussionTo our knowledge, this is the first meta-analysis to explore the association of PD with the risk of GAC. In this study, patients with PD were at risk of developing GAC, which validates the hypothesis that PD is proposed as a potential carcinogenic factor.16 The authors also observed that the risk for GAC continues regardless of the diagnostic method for PD. However, there were differences between populations; Asian patients were at risk of developing GAC associated with PD than Americans and Europeans. It is necessary to point out that the studies had several adjustment variables: Sex; Smoking; Alcohol; socioeconomic status; Intake of vegetables and fruits; BMI; Regular physical activity. Therefore, the present results highlight that periodontal diseases have a significant effect on GAC, further studies are needed to assess how this mechanism occurs and which other microorganisms may be linked to oral-gastric dysbiosis.
Gastric cancer is the leading cause of death among men in South Asian countries.1 This study found an association between periodontal disease and gastric adenocarcinoma in Asian studies, as opposed to American and European studies. Asian populations have polymorphisms of the interleukin genes (IL-17 and IL-10) that increase the risk of gastric cancer, due to their interaction with H. pylori and the habit of smoking.34 These same genetic polymorphisms can cause phenotypic differences in the inflammatory responses in PD, which are important in the individual's sensitivity to the disease, in the progression of the disease or in the response to treatment.35 The prevalence of PD varies between 16 % (Western Pacific region) and 23 % (Africa region), while case numbers reflect the demographic share of the respective regions, with Southeast Asia and Western Pacific regions having the highest number of cases and the Eastern Mediterranean region with the lowest number of PD cases.4
The gold standard for evaluating PD is probing all teeth and radiographic interpretation.36,37 However, in studies conducted with large populations, they are less feasible since they require a big number of trained examiners, high-costly dental equipment, and infection control protocols that demand unpractical execution time.37–39 Self-report and clinical examination are an accessible, reliable, and cost-saving method.39–41 In this meta-analysis, the authors observed an increased risk of PD patients developing GAC, regardless of the diagnostic method used for PD.
This meta-analysis presents limitations. There is a missing information among the studies, such as sex16,26,27,30–33 topography (cardia and non-cardia),16,26,30–33 and different age groups.16,26,30–33 In addition to what studies use as a proxy for PD (gingivitis, periodontitis and tooth loss), therefore, the authors included it in the systematic review. However, it has strength as many participants providing accurate risk estimates, and a high methodological quality of the selected studies. Furthermore, the sensitivity analysis identified that patients with PD may be associated with the development of GAC, regardless of the diagnostic method for PD.
Since there is not enough evidence demonstrating how this association between PD and risk of GAC occurs. Additional studies with more detailed PD data and assessment of the oral microbiome may provide more clarity.
ConclusionThe presence of PD increased the risk of GAC. Several studies suggest that the infectious-inflammatory process of PD can initiate complex reactions involving inflammation mediators and microorganisms that may link the risk of tumor development, therefore there are biological bases to support a relationship between PD and GAC, but more studies are needed to assess the depth of this connection. In addition to considering the screening of patients at potential risk for GAC in the clinical practice of dentists.
PRISMA 2009 checklist statementThe authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to it.
Supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ‒ Brasil (CAPES) ‒ student fellowship by Finance Code 001.