Paracoccidioidomycosis (PCM) is a systemic granulomatous mycosis caused by the fungus Paracoccidioides brasiliensis. Also known as South American blastomycosis and Lutz-Splendore-Almeida disease, PCM is endemic in South and Central America, especially in Brazil, Venezuela, Colombia, Ecuador, and Argentina.1 Its chronic form, which accounts for more than 80% of cases, usually affects men between 29 and 60 years old—predominantly rural workers—and it is characterized by polymorphic lesions that may affect any organ, in particular the skin; the lymph nodes; the lungs; the oral, nasal, and gastrointestinal mucosa; the adrenals; and the central nervous system.1 The genitourinary tract is the least commonly involved system in chronic PCM. In the present paper, we describe two cases of genitourinary PCM located in the penis and the prostate that were treated at our institution, and we review the pertinent literature.
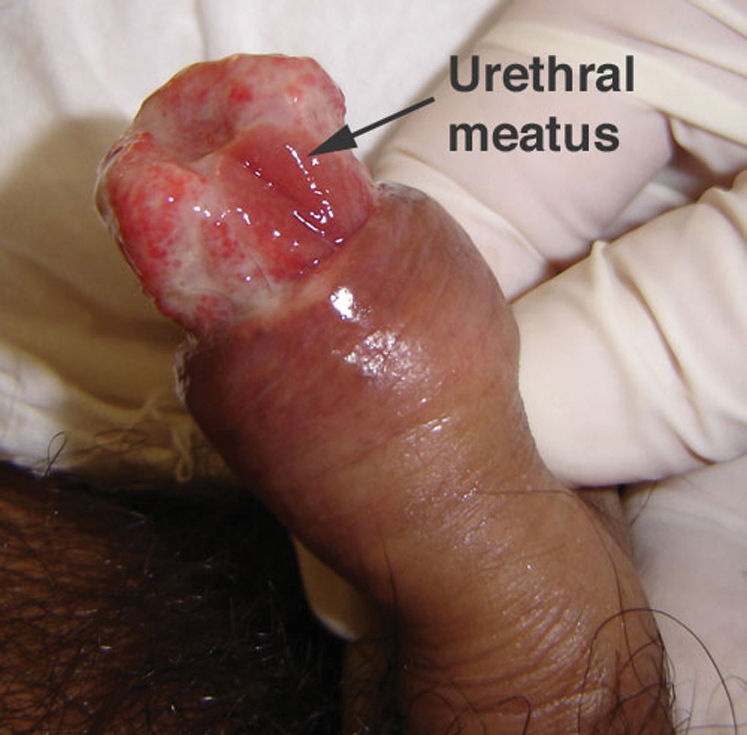
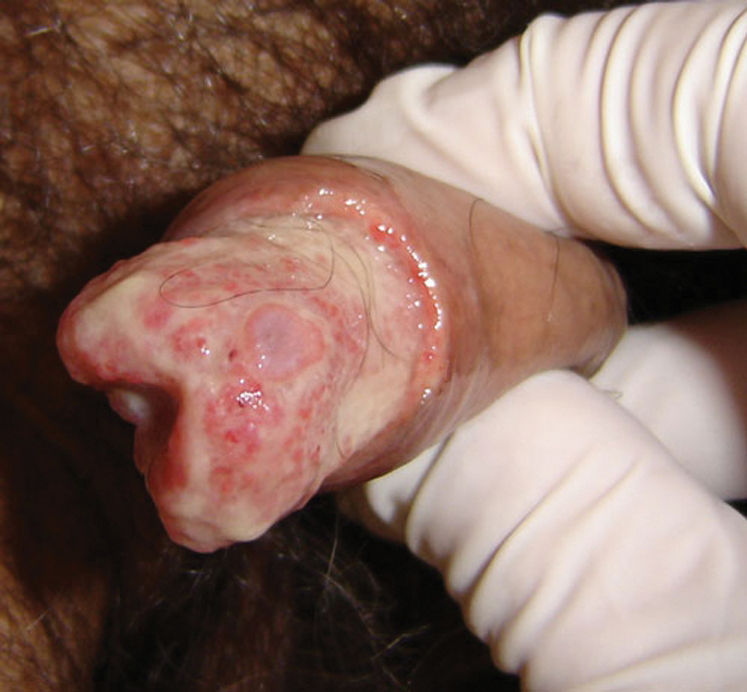
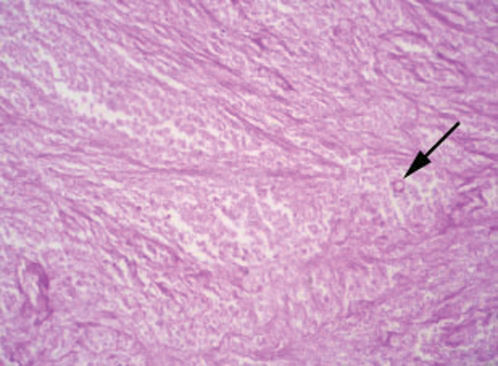
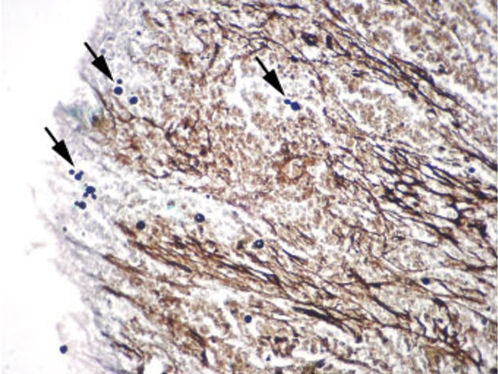
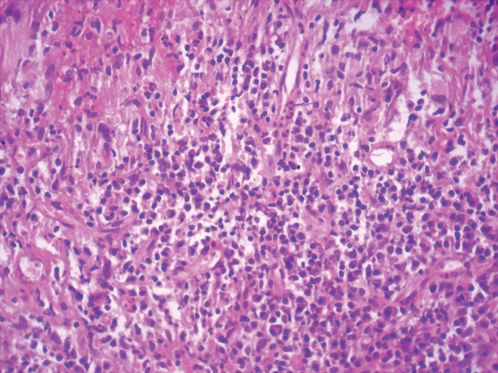
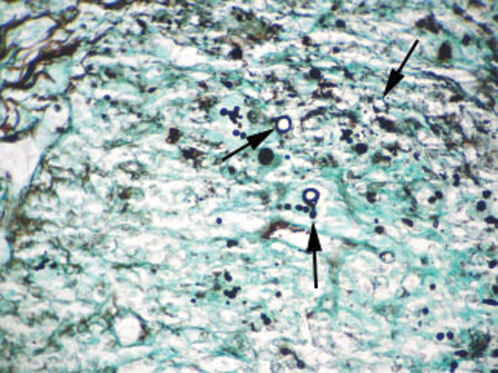
CASE REPORTCase 1A 56-year-old white male presented with a 3-month history of hesitancy, weak urinary stream, intermittency, nocturia, incomplete voiding, terminal dribbling, dysuria, lower abdominal pain, and painful lesions on the penis, along with a lower lip lesion. His history was positive for cigarette smoking, arterial hypertension, and cerebrovascular disease. He had worked in agriculture for 12 years but had been living in urban areas for the past 24 years. On physical examination, the patient had poor hygiene and nutritional conditions, body lesions suggestive of scabies infestation, and a 1.5-cm ulcerated lesion on the lower lip. Pulmonary auscultation revealed a diffuse decrease in breath sounds. A genital exam demonstrated an ulcerated lesion partially exposed on the glans penis caused by poor retractability of the foreskin (Figures 1 and 2); the scrotum and prostate exams were normal. Laboratory tests revealed normal renal function, a negative HIV ELISA-test, a negative urine culture, a negative PPD, and negative acid-fast bacilli in the sputum. Chest radiography showed diffuse and nodular infiltrates. The patient underwent dorsal incision of the foreskin and Foley catheterization, which immediately drained 1,500 cc of concentrated urine. A penile biopsy was positive for PCM (Figures 3 and 4). He underwent antibiotic treatment with sulfamethoxazole (800 mg) and trimethoprim (160 mg) twice a day for 6 months. The Foley catheter was removed after 4 weeks of antibiotic treatment, with satisfactory voiding. Unfortunately, the patient was lost to follow-up before the antibiotic treatment was completed.
A 59-year-old white male living in a rural area presented with a 3-year history of obstructive and irritative lower urinary tract symptoms, which culminated in acute urinary retention requiring emergency suprapubic cystostomy. His medical history was positive for arterial hypertension and pulmonary PCM treated 2 years earlier with sulfamethoxazole/trimethoprim. On physical examination, the left testis was enlarged and painful, with ipsilateral, painless lymph node enlargement. A prostate exam revealed a grade 1 smooth, elastic, and painless prostate. Laboratory tests showed normal renal function, a PSA concentration of 2.47 ng/mL, proteinuria, leukocyturia, a negative urine culture, a negative PPD, and negative acid-fast bacilli in the sputum. With the suspicion of genitourinary PCM, a US-guided prostate biopsy was performed, which revealed granulomatous chronic prostatitis with fungal structures consistent with PCM (Figures 5 and 6). He underwent treatment with itraconazole (200 mg/day) for 6 months, followed by transurethral resection of the prostate, which confirmed complete resolution of PCM upon a pathological examination of the specimen. The cystostomy tube was occluded and removed after satisfactory micturition. Improvement of testicular enlargement with itraconazole provided indirect evidence of testis PCM, but the diagnosis was not confirmed pathologically. At 3 years of follow-up, the patient shows no evidence of recurrence.
Genitourinary PCM is rare, with a clinical incidence of 1.6% to 2% among individuals with chronic PCM. When genitourinary PCM is present, the infection is most common in the scrotum,2-5 the epididymis,2,6 the testis,2,5,7 the prostate,2,6,8,9 and the penis.2 The exact number of cases, however, cannot be precisely assessed because of underreporting, the reporting of cases in non-indexed local journals, nomenclature problems, and non-routine use of mycological tests in patients with suspected infection.
PCM is generally considered to result from the inhalation of spores released into the air, with the lungs being the usual site of primary infection. In the acute/subacute form, superficial and/or visceral lymph node enlargement is the major presentation. In the chronic form, which occurs in 80% to 90% of cases, infection results from reactivation of quiescent lesions. Chronic PCM can manifest after long latency periods, up to 30 years after the individual has left an endemic area.1 The lung is the only organ affected in 25% of chronic PCM cases, while the remaining cases are multifocal, with mucocutaneous lesions in the mouth, nose, ears, pubis, and perineal regions. Primary mucocutaneous involvement is uncommon and may be characterized as an inoculation lesion.4
The age of presentation of genitourinary PCM is often between 30 and 75 years, which is somewhat above the normal range for chronic PCM.3 It is almost exclusively a disease of men. Genitourinary PCM is extremely rare in females because feminine hormones presumably protect women of fertile age.1,10-12 Patients almost always present with multifocal disease, with lung infiltrates or other mucocutaneous lesions suggestive of PCM.2,3 Patients usually present with chronic illness, complaining of anorexia, muscle weakness, and weight loss.2,6,13 As with other chronic PCM manifestations, lymphadenopathy is uncommon in genitourinary PCM.
Scrotal lesions may present as frequently painful erythematous plaques with well-defined edges and as ulcerated, granulomatous, or verrucous lesions that may be associated with lesions in the inguinal, perineal and perianal regions.3-5 Lesions are usually clean, without secondary infection, and with characteristic fine granulations and hemorrhagic dots.1 Scrotal PCM usually results from hematogenic dissemination, as with other forms of chronic PCM, but may occasionally be a consequence of the anal toilet practice of using leaves from contaminated plants.1,4 Diffuse eruptions may simulate syphilis, psoriasis, and lymphomas. If located, they should be differentiated from leishmaniasis, sporotrichosis, tuberculosis, and chromomycosis.1
Unilateral, hardened, painful enlargement of the testicle and/or epididymis are the main manifestation of PCM epididymo-orchitis.2,6 Epididymitis may coexist with a normal ipsilateral testicle.6,14 A fistulous tract draining purulent secretions rich in parasites may be present.1,2 The differential diagnosis includes specific epididymo-orchitis (e.g., tuberculosis, brucellosis) and testicular neoplasms.
Lesions on the penis may involve the external skin and/or the internal aspect of the foreshaft and the glans penis.15 These lesions may be ulcerated, with infiltrated borders and hemorrhagic or seropurulent secretions. They usually do not reproduce the fine microgranulation and hemorrhagic dots observed in mucous membranes. Although penile PCM is usually asymptomatic, the site may be tender, and lower urinary tract symptoms may be present if the infection is located near the external urethral orifice. The differential diagnosis includes penile cancer and ulcerated/papillary sexually transmitted lesions (e.g., HPV, syphilis, genital herpes, chancroid, lymphogranuloma venereum, and donovanosis). There is no evidence that penile PCM may be sexually transmitted because the fungus is not in an infectious state (yeast form) inside humans.1 The infecting form of the fungus is the mycelium form, found only in nature at a temperature of 25°C.
Most lesions in the prostate are asymptomatic and are found during necropsy,9 but they may also cause symptoms of lower urinary tract obstruction.6,8 Prostatic lesions may be palpated in a normal or enlarged prostate as indurated lesions during digital rectal examination, mimicking nonspecific chronic prostatitis or prostate cancer.8 Associated with prostatic PCM, unilateral kidney exclusion may also occur, presumably as a consequence of hematogenous dissemination of the fungus leading to autonephrectomy, similar to the process observed in kidney tuberculosis.8 Prostate PCM has also been described as being involved secondarily via the canalicular path from the epididymis.16
The diagnosis is made by isolation of the fungus by either histopathological, cytopathological, or direct mycological examination after puncture biopsy or culture. A biopsy with hematoxylin-eosin stain shows a granulomatous pattern, with findings of a double-wall parasite with simple or multiple gemmulation. The granuloma is rich in epithelioid and giant cells, some containing variable amounts of parasites. If few in number, the parasites are better visualized through special stains, such as periodic acid-Schiff or Gomori-Grocott. Serology in PCM has applications in diagnosis and follow-up. PCM treatment includes the use of antifungal drugs, nutritional support, the prevention of opportunist diseases, and treatment of eventual sequelae and complications.1
Urinary outflow obstruction is an uncommon presentation of paracoccidioidomycosis. This clinical entity can manifest even long after the patient has left an endemic area and should always be born in mind as a differential diagnosis in the presence of multifocal mucocutaneous, pulmonary, and/or genitourinary lesions.