Multiple endocrine neoplasia type 2 is an autosomal-dominant hereditary cancer syndrome caused by missense gain-of-function mutations of the rearranged during transfection proto-oncogene, which encodes the receptor tyrosine kinase, on chromosome 10. It has a strong penetrance of medullary thyroid carcinomas and can be associated with bilateral pheochromocytoma and primary hyperparathyroidism. Multiple endocrine neoplasia type 2 is divided into three varieties depending on its clinical features: multiple endocrine neoplasia type 2A, multiple endocrine neoplasia type 2B, and familial medullary thyroid carcinoma. The specific rearranged during transfection mutation may suggest a predilection toward a particular phenotype and clinical course of medullary thyroid carcinoma, with strong genotype-phenotype correlations. Offering rearranged during transfection testing is the best practice for the clinical management of patients at risk of developing multiple endocrine neoplasia type 2, and multiple endocrine neoplasia type 2 has become a classic model for the integration of molecular medicine into patient care. Recommendations on the timing of prophylactic thyroidectomy and extent of surgery are based on the classification of rearranged during transfection mutations into risk levels according to genotype-phenotype correlations. Earlier identification of patients with hereditary medullary thyroid carcinoma can change the presentation from clinical tumor to preclinical disease, resulting in a high cure rate of affected patients and a much better prognoses.
Multiple endocrine neoplasia type 2 (MEN2) is an autosomal-dominant hereditary cancer syndrome caused by missense mutations in the RET (REarranged during Transfection) proto-oncogene, and these result in the gain-of-function of the encoding receptor tyrosine kinase (1). The estimated prevalence is 2.5 per 100,000 in the general population. MEN2 shows a high penetrance for medullary thyroid carcinoma (MTC), which is a rare calcitonin (Ct)-secreting tumor derived from thyroid parafollicular or C-cells of the thyroid; these cells are themselves derived from the neural crest. Most patients with MTC have the sporadic (i.e., non-familial) form of the tumor, while 25–30% present with the hereditary form. MEN2 syndrome occurs in three clinically distinct varieties with variable penetrance of MTC, pheochromocytoma, and hyperparathyroidism. Early identification of patients with MTC changes the presentation from a clinically evident tumor to a preclinical disease, resulting in a higher cure rate of the affected patients with much better prognoses (2). Each variant of MEN2 results from a different mutation of the RET gene with good genotype-phenotype correlations.
Phenotype: clinical description of MEN2Three distinct clinical subtypes of MEN2 have been characterized (Table 1). They differ with respect to incidence, genetics, age-related penetrance, association with other diseases, aggressiveness of MTC, and prognosis (3),(4). MEN2A syndrome (OMIM#171400) is characterized by the presence of MTC, bilateral pheochromocytoma and/or multiple tumors of the parathyroid glands (primary hyperparathyroidism) within a single patient or family. It is the most common form of MEN2 syndrome, representing 55% of cases (5). The frequency of MTC is >90% among patients with MEN2A, while the frequencies of pheochromocytoma and multiple parathyroid gland hyperplasia are 40–50% and 10–20%, respectively. MTC is generally the first manifestation of MEN2A and presents when patients are between 5 and 25 years of age. Rare variants of MEN2A exist, including MEN2A with cutaneous lichen amyloidosis (this skin lesion is located over the upper portion of the back) and MEN2A/familial MTC (FMTC) with Hirschsprung's disease (see below).
Clinical classification of MEN2 and FMTC, and occurrence of MTC, associated tumors and other diseases.
| Subtype | Percentage of total cases | Typical age of onset (years) | MTC (%) | Pheo (%) | HPT (%) | Associated diseases |
|---|---|---|---|---|---|---|
| MEN2A | 56 | 10 | 100 | 50 | 25 | Cutaneous lichen amyloidosis, Hirschsprung disease |
| MEN2B | 9 | 2 | 100 | 50 | Ganglioneuromatosis, marfanoid habitus | |
| FMTC | 35 | 30 | 95 | Rare |
FMTC: familial medullary thyroid carcinoma. HPT: primary hyperparathyroidism. MEN2: multiple endocrine neoplasia type 2. MTC: medullary thyroid carcinoma. Pheo: pheochromocytoma.
MEN2B (OMIM#162300) is characterized by the association between MTC, pheochromocytoma, the absence of hyperparathyroidism, and visible physical stigmata such as raised bumps on the lips and tongue (caused by cutaneous neuromas), ganglioneuromas of the intestine, and asthenic marfanoid body habitus with skeletal deformations and joint laxity. Pheochromcytomas occur in 50% of individuals with MEN2B, approximately half of these are multiple and often bilateral; the development of mucosal neuromas on the tongue and lips (bumpy lips) (resulting in a distinctive facial appearance), together with a marfanoid body habitus, allows for diagnosis at a first glance. Early diagnosis by clinical means is difficult because of the gradual development of the typical clinical appearance during childhood. Diffuse ganglioneuromatosis of the gastrointestinal tract is responsible for the megacolon, constipation and diarrhea that often present. Because of their similar clinical presentations, the clinician should carefully differentiate the diagnosis of Hirschsprung disease as a result of aganglioneuromatosis from MEN2B. Brauckhoff et al. reported that 86% of patients with MEN2B demonstrate an inability to cry tears (6). It is the rarest form of MEN2 and accounts for 5–10% of MEN2 cases. Patients with MEN2B typically present with MTC during the first year of life and have a more aggressive form of MTC with higher morbidity and mortality rates than patients with MEN2A (4). Patients with MEN2B often do not have a family history of the disease; in more than 50% of cases the syndrome is due to a de novo germline RET mutation.
FMTC (OMIM#155240) is characterized by a strong predisposition to MTC in families with a very low incidence of other endocrinopathies related to MEN2. It is clinically diagnosed in families with four or more cases of MTC. It is viewed as a phenotypic variant of MEN2A with decreased penetrance of pheochromocytoma and primary hyperparathyroidism. It has been diagnosed more frequently in recent years and is reported to account for 35–60% of all MEN2 cases (5,7–9). Regarding MTC in FMTC, penetrance is lower and the clinical course is more benign than in MEN2A and MEN2B, with a late onset or no clinically manifesting disease and the prognosis is relatively good. Therefore, a family history is often inadequate for establishing familial disease.
Medullary thyroid carcinoma (MTC)MTC originates from neural crest-derived Ct-producing cells (C-cells). Hereditary MTC characteristically presents as a multifocal process with C-cell hyperplasia (CCH) as the precursor lesion to MTC. Only familial primary CCH is a preneoplastic lesion. Secondary CCH, which has been associated with chronic lymphocytic thyroiditis, hypergastrinemia, other follicular-cell-derived thyroid tumors and even aging, has a much lower, if any, potential for malignancy (10). The time frame for the progression from CCH to microscopic carcinoma in MEN2 remains unclear, but it may take years; the age of transformation from CCH to MTC varies with different germline RET mutations. MTC is diagnosed when nests of C-cells extend beyond the basement membrane and infiltrate and destroy thyroid follicles. MTC in MEN2 patients is multicentric and concentrated in the upper third of the thyroid gland. The peak incidence of sporadic MTC occurs in the fifth decade of life, while hereditary MTC can be diagnosed earlier, depending on the availability of genetic and biochemical screenings. The mode of discovery of MTC has changed, based on the use of specific strategies: serum Ct screening is used for patients with thyroid nodules (11) and genetic screening for RET proto-oncogene mutations is used for all patients with MTC (9). The earlier identification of patients with MTC has altered the presentation from symptomatic tumors to a preclinical disease, resulting in a much better prognosis with a higher cure rate. The average 5-year survival for MTC is 83%, which is lower than that for papillary and follicular thyroid cancers. The most important predictor of survival is the tumor stage at diagnosis. Metastasis may be found first in the central, lateral cervical and mediastinal lymph nodes of the neck in 30% of patients with MTC <1 cm in diameter. Metastases outside the neck and mediastinum may develop during the course of the disease, predominantly in the lung, liver, and bone.
The primary secretory product of MTC is Ct, which serves as a tumor marker for MTC. The normal concentration of mature Ct is <10 pg/ml and after stimulation it is <100 pg/ml. The Ct secretagogues are pentagastrin (0.5 μg/kg body weight), which is no longer available in the USA, and calcium (0.25 mg/kg body weight) (12). Basal Ct levels are elevated in some patients with renal insufficiency, autoimmune thyroid disorders or hypergastrinemia. This has to be taken into account when discussing elevated Ct levels. Either basal or stimulated plasma Ct levels using pentagastrin or calcium are elevated in virtually all patients with MTC. Basal Ct concentrations usually correlate with tumor mass and are almost always high in patients with palpable tumors (13). Similarly, following surgery to remove a tumor, elevated plasma Ct levels are indicative of persistent or recurrent disease.
PheochromocytomaThe clinical manifestation of pheochromocytoma associated with MEN2 is similar to that seen in sporadic cases, with signs and symptoms such as headache, palpitations, nervousness, tachycardia, and hypertension. Measurement of plasma and/or 24-h urinary excretion of catecholamine metabolites (e.g., epinephrine, norepinephrine, metanephrine and normetanephrine) should be performed. Once the biochemical diagnosis is made, imaging studies, such as abdominal magnetic resonance imaging and/or computed tomography, in addition to metaiodobenzylguanidine scanning and/or fluorodopamine positron emission tomography, are appropriate. Pheochromocytoma in MEN2 is almost always located in the adrenal and is almost always benign. The possibility of bilateral disease must be carefully evaluated. The presence of pheochromocytoma must be ruled out prior to any surgical procedure because if it is untreated it can be lethal. Endoscopic adrenal-sparing surgery has become the method of choice. The penetrance of pheochromocytoma among different MEN2 kindreds depends on specific RET germline mutations (see below) (14). Pheochromocytoma usually becomes evident approximately 10 years later than the manifestation of MTC. Therefore, it is usually identified when screening patients with MEN2, often before clinical symptoms present. Biochemical screening for pheochromocytoma should be performed annually in MEN2 patients depending on their genotype (see below), beginning at 8 years of age for carriers of RET mutations associated with MEN2B and codon 634 mutations and by the age of 20 years for carriers of other codon mutations. Because of the high risk to the fetus and the mother, women with a RET mutation associated with MEN2 should be screened for pheochromocytoma before pregnancy.
Primary HyperparathyroidismPrimary hyperparathyroidism is often clinically occult and does not differ from that seen in patients with mild sporadic primary hyperparathyroidism. It has been reported in 10–25% of patients with MEN2A, but is rarely the first manifestation of this syndrome (15),(16). Diagnosis is established by detecting high concentrations of intact parathyroid hormone in the presence of hypercalcemia, usually after the third decade of life. Pathological findings show chief cell hyperplasia involving multiple glands and sometimes multiple adenomas. Annual measurement of the serum calcium concentration and parathyroid hormone in gene carriers is probably adequate for screening purposes, and should begin by age 8 years for carriers of RET mutations in exon 10 and 11 and by age 20 years for carriers of other MEN2A RET mutations. The goal for MEN2 patients with primary hyperparathyroidism is to excise the enlarged glands and to leave at least one normal parathyroid gland intact. If the glands are all enlarged, a subtotal parathyroidectomy or total parathyroidectomy with autotransplantation should be performed.
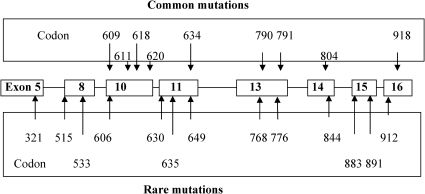
Genotype of MEN2: RET proto-oncogene structure, function and abnormalitiesThe genes responsible for MEN2 were found to be localized in centromeric chromosome 10 (10q11.2) by genetic linkage analysis in 1987. Subsequently, point mutations of the RET (REarranged during Transfection) proto-oncogene were identified in MEN2A, MEN2B, and FMTC in eight exons located near this region (Figure 1) (17),(18). Analysis of RET in families with MEN2A and FMTC revealed that nearly all of these families have germline mutations, and that only those family members with the germline missense mutations have the disease. This discovery prompted major advances in the understanding of the molecular and genetic basis of MTC, and has significantly changed the clinical management of families with hereditary tumors. Missense mutations have been reported in exons 5, 8, 10, 11, and 13–16 of RET (http://arup.utah.edu/database/MEN2/MEN2_welcome.php) (Figure 1) (5). Genetic testing detects about 98% of mutation carriers and is considered to be standard care for all first-degree relatives of patients with newly diagnosed MTC. It is also recommended for all patients with MTC, regardless of other features or family history, especially those with clinical features that are indicative of MEN2 and those with primary CCH. About 4–10% of patients with apparently sporadic MTC have germline mutations in the RET proto-oncogene and, consequently, present with the hereditary form (8).
The RET gene consists of 21 exons and encodes a receptor tyrosine kinase that appears to transduce growth and differentiation signals in several developing tissues, including those derived from the neural crest. The transcript length is 5659 base pairs and is translates to a 1114 amino acid-residue protein. The RET protein consists of an extracellular segment with a ligand-binding domain, a cadherin (Ca2+-dependent cell adhesion)-like domain, and a cysteine-rich domain that is positioned near the cell membrane. The RET protein has a single transmembrane domain and an intracellular segment with two tyrosine kinase subdomains, TK1 and TK2. It is activated by ligand-induced dimerization (19). RET binds ligands of the glial-derived neurotrophic factor (GDNF) family in conjunction with a co-receptor, designated GDNF-family receptor α, to a complex that triggers autophosphorylation and intracellular signaling. RET is produced in neuroendocrine cells, including C-cells of the thyroid (the precursors of MTC) and pheochromocytomas.
Hereditary MTC is caused by autosomal-dominant gain-of-function mutations in the RET proto-oncogene. Mutation of the extracellular cysteine in codon 634 in exon 11 causes ligand-independent dimerization of receptor molecules, enhanced phosphorylation of intracellular substrates and cell transformation. Mutation of the intracellular tyrosine kinase (codon 918) has no effect on receptor dimerization but does cause constitutive activation of intracellular signaling pathways and also results in cellular transformation. There is a significant age-related progression from CCH to MTC that correlates with the transformative capacity of the particular RET mutation (2). Mutations that strongly activate the RET proto-oncogene are associated with a more aggressive form of MTC that presents earlier, and mutations that provide weaker RET activation result in a less aggressive form of the disease that presents later. On the basis of this finding, a risk-stratification strategy has been developed (see below). MTC is generally the first neoplastic manifestation seen in patients with MEN2A because of its earlier age of onset and higher rate of penetrance compared with pheochromocytoma or parathyroid hyperplasia. This indicates that C-cells are more susceptible to oncogenic RET activation than adrenal medullary or parathyroid cells.
RET has also been implicated in congenital aganglionosis (absence of enteric nerve cells) in the gastrointestinal tract (Hirschsprung disease). The lack of neuroenteric plexi impairs smooth muscle activity of the intestines (particularly the colon), resulting in refractory constipation. Up to 50% of familial cases and up to 35% of simplex cases (i.e., a single occurrence in a family) of Hirschsprung disease are caused by germline loss-of-function mutations in the RET proto-oncogene (20). There are some families and individuals harboring germline RET mutations in exon 10 that co-segregate with MEN2A/FMTC and Hirschsprung disease. Because of a seemingly similar clinical presentation, clinicians should be careful to differentiate the diagnosis of Hirschsprung disease from the constipation/obstipation that result from ganglioneuromatosis associated with MEN2B.
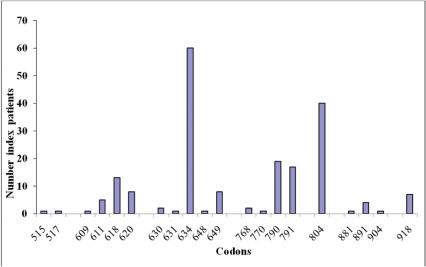
Genotype-phenotype correlations in MEN2Clear associations have been documented between specific RET mutations (genotype) and the age of onset and aggressiveness of MTC and the presence or absence of other endocrine neoplasms (phenotype), such as pheochromocytoma or hyperparathyroidism (Table 2) (2),(21),(22). Some overlap exists between RET mutations and the resulting clinical subtype of MEN2. Approximately 98% of families with MEN2A have a RET mutation in exon 10 or 11. Mutations in the cysteine of codon 634 occur in about 87% of families; 95% of families with FMTC have an identifiable RET mutation. These mutations typically occur in one of the five cysteine residues (codons 609, 611, 618, 620, and 634 in exons 10 and 11) or in exons 5, 8, and 13–16, with an important minority affecting codons 790, 791, 768, and 804; 95% of individuals with the MEN2B phenotype have a single-point mutation in the tyrosine kinase domain of the RET gene at codon 918 in exon 16, and this substitutes a threonine for methionine (M918T) (Figure 2). A second mutation at codon 883 in exon 15, A883F, has been identified in several affected individuals without a M918T mutation (4). In addition to these well-known and clinically well-characterized mutations, more than 140 RET variants are known. Half of these variants are pathogenic, and the other half are benign or uncertain variants (http://arup.utah.edu/database/MEN2/MEN2_welcome.php).
Management of patients with different RET mutations (4).
| Characteristic/management | Codons 321, 515, 533, 600, 603, 606, 635, 649, 666, 768, 776, 790, 791, 804, 819, 833, 844, 861, 891, 912 | Codons 609, 611, 618, 620, 630, 631 | Codon 634 | Codons 918, 883 |
|---|---|---|---|---|
| ATA risk level (2009)a) | A | B | C | D |
| MEN2 subtype | FMTC | FMTC/MEN2A | MEN2A | MEN2B |
| MTC aggressiveness | Moderate | High | Higher | Highest |
| MTC age of onset | Adults | 5 years | Before the age of 5 years | First year of life |
| Timing of prophylactic thyroidectomy | When calcitonin rises/age 5 or 10 years | 5 years | Before the age of 5 years | First months of life |
| Screening for Pheo | Start at 20 years, periodically | Start at 20 years, annually | Start at 8 years, annually | Start at 8 years, annually |
| Screening for HPT | Start at 20 years, periodically | Start at 20 years, periodically | Start at 8 years, annually | - |
ATA: American Thyroid Association. FMTC: familial medullary thyroid carcinoma. HPT: primary hyperparathyroidism. MTC: medullary thyroid carcinoma. Pheo: pheochromocytoma.
In newly detected germline RET sequence changes it is mandatory to clarify if the “mutation” is causative of MEN2 and if it segregates with the MEN2 disease symptoms within a family. The web-based Associated Regional and University Pathologists (ARUP) online Scientific Resource RET database (http://arup.utah.edu/database/MEN2/MEN2_welcome.php) uses the following classification definition: mutation, polymorphism, and variant of unknown significance (VUS). Mutation is defined as causative for the disease and segregates with the disease if the following conditions are met: (1) if there are at least two affected family members and at least one has MTC and one other family member has one clinical feature of MEN2; (2) if one patient has MTC and another has MEN2 clinical features; or (3) if at least three unrelated individuals with MTC have the same germline RET sequence variant. Polymorphisms are harmless germline RET sequence changes that are not causative of MEN2, and they include G691S, L769L, S836S, S904S and intron 14 c.2608-24G>A. It is speculated that some of the normal allelic variants may be modifying factors in patients with pathologic RET mutations (23-25). VUS are RET sequence changes in which there is not enough clinical evidence to indicate a causative role, so further clinical studies, in vitro experiments (e.g., kinase activity assay), and prediction algorithms are necessary to clarify whether the sequence change is a mutation or a polymorphism. One example of this is the S649L and Y791F RET germline sequence variants that have been described and verified as mild mutations by some (26-28) and as non-pathogenic by others (29). Using the Bayes classification to predict the disease associations of uncertain gene variants into categories of benign and pathogenic, amino acid-substitution penalties, structural disruption, sequence homology (e.g., ortholog conservation) or neural nets, the RET Y791N sequence changes are classified as pathogenic (30). Further clinical studies are necessary to classify VUS as deleterious mutations before guiding therapy by the codon positions of these RET mutations.
Clinical implications of genotype-phenotype correlationsPatients with a codon 918 mutation and MEN2B are at high risk of aggressive MTC occurring at a young age (31). In contrast, patients with codon 791 mutations are at a relatively low risk of aggressive disease and develop slow-growing tumors as a late manifestation (27). According to recent data, membrane proximity of the mutation seems to be an important determinant of tumor development in carriers of RET mutations in exon 10 (32),(33). These genotype-phenotype correlations between mutation, age of onset, tumor aggressiveness, and onset of pheochromocytoma and parathyroid disease may be used to predict the phenotype and make recommendations regarding the ages at which to perform prophylactic thyroidectomy and begin biochemical screening for pheochromocytoma and hyperparathyroidism (Table 2). This is particularly true in presymptomatic RET mutation carriers because a prophylactic thyroidectomy should be performed before the development of MTC, or at least when the MTC is confined within the thyroid and before the spread of disease beyond the gland. The multicentric and bilateral nature of hereditary MTC implicates a total thyroidectomy.
Recommendations for the timing of prophylactic thyroidectomy and the extent of surgical resection are based on a model that utilizes these genotype-phenotype correlations to stratify mutations into risk levels (A–D) (Table 2) (4). Patients with American Thyroid Association (ATA) A mutations (exon 5, codon 321; exon 8, codons 531, 515 and 533; exon 10, codons 600, 603 and 606; exon 11, codons 649 and 666; exon 13, codons 768, 777, 790 and 791; exon 14, codons 804, 819, 833 and 844; and exon 15, codons 866, 891 and 912), which mainly include phenotypic FMTC, are at the lowest risk for the development and aggressiveness of MTC; patients with ATA B mutations (exon 10, codons 609, 611, 618 and 620; and exon 11, codons 630, 631 and 633) are at intermediate risk; patients with ATA C mutations, the classical MEN2A mutation (exon 11, codon 634) are at a higher risk than patients with ATA B mutations; and patients with ATA D mutations have the typical MEN2B mutation (exon 15, codon 883; and exon 16, codon 918) and are at the highest risk of developing MTC with a high rate of growth.
In the cases of these higher risk mutations, thyroidectomy is recommended before 5 years of age for patients with ATA C mutations and as early as possible, preferably within the first year after birth, for patients with ATA D mutations (4). For patients with ATA A mutations, there are various recommendations regarding the appropriate age at which to perform prophylactic surgery: some authors suggest thyroidectomy at 5 years of age, some at 10 years, while others, including the present authors (27), suggest that surgery may be postponed until an abnormal C-cell stimulation test result is obtained (i.e., an abnormal Ct response to pentagastrin or calcium stimulation). However, further studies, particularly regarding rare and/or newly diagnosed mutations (26), are necessary before general recommendations can be made. In order to avoid complications such as recurrent laryngeal nerve injuries or hypopathyroidism, the operation should be performed by surgeons who perform a high volume of this type of procedure.
The association between disease phenotype and RET genotype may also have important implications for the management of clinical presentations other than MTC in MEN2 patients and their families. If the genotype could be fully correlated with certain phenotypic features, then a clinician could use a patient's genotype to decide whether intense screening for pheochromocytoma or HPT is necessary in those patients with mutations associated with a higher risk of disease (Table 2). Pheochromocytoma is associated with exon 634 and 918 mutations in approximately 50% of patients, with exon 10 mutations (codons 609, 611, 618 and 620) in up to 17% of patients (33), and rarely with mutations in exons 13–15 (codons 791 and 804; Tables 1 and 2 (14),(34). Hyperparathyroidism in MEN2A is most commonly associated with codon 634 mutations and C634R in particular (35).
All cases of MEN2A with Hirschsprung disease are associated with mutations in exon 10 (codons 609, 611, 618, and 620); in an unselected, multinational study on carriers of exon 10 mutations, 7.5% of informative carriers were found to also have a Hirschsprung disease phenotype (33). MEN2A with cutaneous lichen amyloidosis is associated with mutations in codon 634 (36).
Perspectives: screening and genetic counselingMEN2 is a monogenic disorder and, according to its autosomal-dominant pattern of inheritance, each affected individual has a 50% probability of transmitting the defective gene to progeny, independent of sex. Predictive DNA tests are available; therefore, it is worthwhile to screen both first- and second-grade family members. In clinically demonstrable MTC, the specific RET mutation gives information on the risk of developing pheochromocytoma and HPT, the aggressiveness of MTC and clinical prognosis. By screening family members, genetic testing allows for early treatment and, thereby, offers the chance of prophylactic thyroidectomy and curing the patient of MTC.
From a medical point of view, MEN2 offers a unique model of how to use genetic information to cure a patient with a hereditary cancer syndrome. From the point of view of the patient, there is a loss of privacy and autonomy and a feeling of stigmatization and discrimination. There appears to be a threshold for patients to inform their relatives about this hereditary disease. The confidentiality of genetic testing must be absolute, with no exception; therefore, the duty to inform relatives who may be at risk depends on the moral obligation of the patient. However, many guidelines do allow for the disclosure of results to at-risk individuals without the patient's consent when the information that will be disclosed will prevent serious harm (4). Our social attitude to rare hereditary diseases has to be reconsidered. We need precise laws to prevent discrimination concerning full insurance coverage and employment. Patient interest groups may be able to providing information, support during times of emotional distress and stand up for common political and social aspects.
Early identification of patients with hereditary MTC using DNA screening changes the presentation from a clinical tumor (index case) to a preclinical disease (screening case), resulting in a high cure rate of the affected patients by using presymptomatic treatments, improved life expectancy and quality of life.
No potential conflict of interest was reported.
Raue F outlined the paper, wrote all of the sections except the genotype-phenotype section, prepared the tables and figures, and checked the final version. Frank-Raue K wrote the genotype-phenotype section, updated the references and read the proof of the manuscript.