Renal artery stenosis can lead to renovascular hypertension; however, the detection of stenosis alone does not guarantee the presence of renovascular hypertension. Renovascular hypertension depends on activation of the renin-angiotensin system, which can be detected by functional tests such as captopril renal scintigraphy. A method that allows direct measurement of the baseline and post-captopril glomerular filtration rate using chromium-51 labeled ethylenediamine tetraacetic acid (51Cr-EDTA) could add valuable information to the investigation of hypertensive patients with renal artery stenosis. The purposes of this study were to create a protocol to measure the baseline and post-captopril glomerular filtration rate using 51Cr-EDTA, and to verify whether changes in the glomerular filtration rate permit differentiation between hypertensive patients with and without renal artery stenosis.
METHODS:This prospective study included 41 consecutive patients with poorly controlled severe hypertension. All patients had undergone a radiological investigation of renal artery stenosis within the month prior to their inclusion. The patients were divided into two groups: patients with (n=21) and without renal artery stenosis, (n=20). In vitro glomerular filtration rate analysis (51Cr-EDTA) and 99mTc-DMSA scintigraphy were performed before and after captopril administration in all patients.
RESULTS:The mean baseline glomerular filtration rate was 48.6±21.8 ml/kg/1.73 m2 in the group wuth renal artery stenosis, which was significantly lower than the GFR of 65.1±28.7 ml/kg/1.73m2 in the group without renal artery stenosis (p=0.04). Captopril induced a significant reduction of the glomerular filtration rate in the group with renal artery stenosis (to 32.6±14.8 ml/ kg/1.73m2, p=0.001) and an insignificant change in the group without RAS (to 62.2±23.6 ml/kg/1.73m2, p=0.68). Scintigraphy with technetium-99m dimercapto-succinic acid (DMSA) did not show significant differences in differential renal function from baseline to post-captopril images in either group.
CONCLUSIONS:Captopril induced a decrease in the GFR that could be quantitatively measured with 51Cr-EDTA. The reduction is more pronounced in hypertensive patients with RAS.
Renovascular hypertension (RVH) is a potentially curable type of hypertension with an estimated prevalence of 3–5% in hypertensive patients.1,2 RVH develops from the activation of the renin-angiotensin system due to renal arterial stenosis (RAS)3 and subsequent vasoconstriction and retention of sodium and water.
RAS can be detected by arteriography and by other noninvasive imaging methods, such as magnetic resonance angiography (MRA), computed tomography angiography (CTA), and duplex ultrasound.4 The detection of stenosis alone does not guarantee the presence of RVH and may not be sufficient for defining a particular prognosis and treatment.
Despite marked technical advances in the endovascular treatment of renal artery occlusive disease, the selection of patients who would benefit from surgical bypass or endovascular therapy remains controversial.5 Clinical improvement after vascular intervention is considered diagnostic for RVH, thereby reinforcing the finding that currently available non-invasive techniques are not sufficiently sensitive and specific. Therefore, there is an increasing interest in developing tests to evaluate the functional consequences of RAS to identify patients who may benefit from dilation procedures.6
Captopril renal scintigraphy is used for the diagnosis of RVH7–9 and is a predictor of blood pressure reduction after surgery or angioplasty. Administration of an angiotensin converting enzyme (ACE) inhibitor leads to a decrease in glomerular filtration pressure, a prolonged transit time of tubular agents (99mTc-MAG3 or EC) and decreased uptake of glomerular agents (99mTc-DTPA). Captopril renal scintigraphy analysis is based on characterization of renal function deterioration when compared to a baseline study, as a decreased GFR is indirectly reflected in time-activity curves.
51Cr-EDTA clearance provides an accurate and objective measurement of the GFR,10 and it can be used to quantify renal function deterioration induced by ACE inhibitors. A simplified method for direct measurement of baseline and post-captopril GFR using 51Cr-EDTA could add valuable information to the investigation of hypertensive patients with RAS, although scintigraphy would still be needed to assess differential renal function. Assuming that hypertensive patients with RAS have a higher probability of developing RVH than patients without RAS, we hypothesized that an effective test would show a greater decrease in GFR measurements in the first group of patients.
OBJECTIVESThe purpose of this study was to create a one-day protocol to measure baseline and post-captopril GFR with 51Cr-EDTA.
METHODSPatientsFrom March 2007 to April 2009, 41 consecutive patients with poorly controlled severe hypertension were prospectively included in the study. Patients were recruited from either the Clinical Hospital Nephrology Department or from the Heart Hospital Hypertension Clinic, both of which are tertiary hospitals. Patients with chronic renal insufficiency (those undergoing dialysis or with a Cockcroft-Gault estimated renal function below 15/ml/min) and women who were pregnant or lactating were excluded.
All patients had undergone a radiological investigation of RAS within a month prior to their inclusion in the study. The patients were divided into two groups: hypertensive patients with RAS and hypertensive patients without RAS.
Clinical data recorded for all patients included: age, sex, time of hypertension onset, blood pressure (BP) level, antihypertensive drugs taken at the time of the study, other atherosclerotic risk factors (dyslipidemia, diabetes, smoking), and previous history of myocardial infarction (MI) or cerebral ischemia (CI).
The study was approved by the Hospital Ethics Committee, and all patients signed informed consent forms.
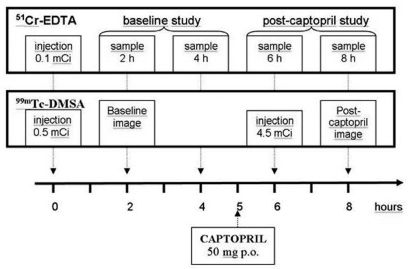
ProceduresBaseline and post-captopril GFRPatients were instructed to stop taking antihypertensive drugs (including ACE inhibitors) for three to five days before the study. A single dose of 3.7 MBq (100 μCi) of 51Cr-EDTA, in a volume of one ml, was injected into an antecubital vein. The exact injected dose was determined by weighing the syringe before and after the injection on a high precision analytic balance. Accurately timed, nine-ml blood samples were drawn from the opposite arm at two, four, six, and eight hours after the injection. Captopril (50 mg) was orally administered five hours after 51Cr-EDTA administration, with blood pressure monitoring conducted every fifteen minutes for one hour thereafter.
To measure radioisotope activity, the blood samples were centrifuged at 3600 rpm for 10 minutes, and three ml of plasma was measured in a well counter calibrated to the energy of chromium-51 (320 keV). Each sample, including a three-ml aliquot of a reference solution of 3.7 MBq (100 μCi) 51Cr- EDTA diluted to 500 ml in saline, was counted for five minutes.
The first two plasma samples (two and four hours) were used to calculate the pre-captopril GFR, and the last two samples (six and eight hours) were used to calculate the post-captopril GFR. Plasma clearance rates were calculated by the slope-intercept method using a single-compartment model. Brochner-Mortensen’s method was used to correct the systematic error of the slope-intercept technique. The plasma clearance of 51Cr-EDTA was corrected for a 1.73 m² body surface.
Evaluation of individual renal functionAn initial dose of 18.5 MBq (0.5 mCi) of 99mTc-DMSA was simultaneously injected with 51Cr-EDTA. After two hours, anterior and posterior abdominal images were obtained for two minutes using a dual-headed gamma camera (ECam - Siemens; Hoffman Estates, Illinois, USA). A second dose of 167 MBq (4.5 mCi) of 99mTc-DMSA was injected one hour after captopril administration, and a second set of images was acquired at two hours post-administration. Differential renal function was semi-quantitatively estimated in both the baseline and the post-captopril studies by determining the geometric means of the anterior and posterior counts in the right and left kidney regions of interest (Figure 1).
Follow-up of patientsThe group of patients with RAS were followed up to 663 days after their inclusion in the study. BP level and antihypertensive drug classes in use six months after the initial evaluation were used to classify patients as either responders or non-responders to the therapy according to the Guidelines for the Reporting of Renal Artery Revascularization in Clinical Trials.11 Improvement in the control of hypertension was defined by a diastolic pressure lower than 90 mmHg and/or a systolic BP lower than 140 mmHg upon use of the same or a less complex scheme of therapy (lower number of antihypertensive drug classes or dosages) or by a reduction in diastolic blood pressure of at least 15 mmHg.
Data analysisPatient demographics were recorded, and the GFRs before and after captopril administration were compared. The mean, standard deviation, minimum and maximum values were determined for quantitative variables. Absolute and relative frequencies of qualitative variables were also calculated. The Student’s t test (unpaired) was employed to compare the quantitative variables, and categorical variables were compared by means of Fisher’s exact test. A p-value < 0.05 was considered significant.
RESULTSThe group of patients with RAS included 21 patients (12 male, nine female). RAS was confirmed by either arteriography or MRA (in 18 and 3 cases, respectively). The mean±SD age was 62.1±9.1 years (range 42–78), and the time of hypertension onset was 16.2±12.0 years. Patients presented with a mean±SD systolic blood pressure of 161±23 mmHg and a mean±SD diastolic BP of 91±14 mmHg. The mean number (±SD) of antihypertensive drugs in use was 3.9 ±1.4.
The group of patients without RAS included 20 patients (11 male, nine female). A non-stenotic renal artery was confirmed by arteriography in 16 cases and MRA and CTA in the remaining four cases. The mean±SD age was 53.1±10.3 years (range 37–77) and the time of hypertension onset was 12.7±9.4 years. The group of patients without RAS presented with a mean±SD systolic blood pressure of 167±23 mmHg and a mean±SD diastolic BP of 98±14 mmHg. The mean number (±SD) of antihypertensive drugs in use was 4.6±0.9.
The group of patients with RAS were significantly older than those without RAS (p-value 0.005), with no statistically significant difference in the time of hypertension onset (p=0.3), systolic blood pressure (p=0.44), diastolic blood pressure (p=0.13), or number of antihypertensive drugs in use (p=0.08). There were no significant differences between groups regarding the prevalence of other atherosclerotic risk factors (diabetes and dyslipidemia) or of previous ischemic lesions (MI or CI) (p>0.1). Clinical and laboratory characteristics of patients with and without RAS are summarized in Table 1.
Clinical and laboratorial characteristics of patients in Groups RAS and no-RAS.
| CLINICAL CHARACTERISTICS | Group RAS (n=21) | Group no-RAS (n=20) |
| Age | 62.1±9.1 years | 53.1±10.3 years |
| time of onset of hypertension | 16.2±12.0 years | 12.7±9.4 years |
| Systolic Blood Pressure | 161±23 mmHg | 167±23 mmHg |
| Diastolic Blood Pressure | 91±14 mmHg | 98±14 mmHg |
| Number of medications in use | 3.9 ±1.4 | 4.6±0.9 |
| Diabetes | 9 (42.8%) | 8 (40%) |
| Dyslipidemia | 20 (95%) | 18 (90%) |
| Smoking (current) | 4 (19%) | 6 (30%) |
| Smoking (previous) | 12 (57%) | 5 (25%) |
| Myocardial infarction | 5 (23.8%) | 6 (30%) |
| Cerebral ischemia | 2 (9.5%) | 5 (25%) |
| LABORATORY TEST RESULTS | ||
| Creatinine (mg/dl) | 1.6 ± 0.7 | 1.4 ±0.8 |
| Urea (mg/dl) | 58.0 ±28.1 | 43.8 ±21.6 |
| Cholesterol (mg/dl) | 188.8 ±48.8 | 205.8 ±42.6 |
| Triglycerides (mg/dl) | 180.6 ±145.1 | 154.9 ±83.4 |
| Blood glucose (mg/dl) | 110.0 ±29.3 | 142.4 ±70.5 |
The mean baseline GFR was 48.6±21.8 ml/kg/1.73 m2 in the group of patients with RAS, which was significantly lower than the GFR of 65.1±28.7 ml/kg/1.73 m2 in the group without RAS (p=0.04). Captopril induced a significant reduction of GFR in the group of patients with RAS (to 32.6±14.8 ml/ kg/1.73 m2, p=0.001) and a non-significant change in the group without RAS (to 62.2±23.6 ml/kg/1.73 m2, p=0.68). The percentage decrease in GFR was also greater in the group of patients with RAS than in the group without RAS (33% and 4.5%, respectively, p=0.007). Baseline and post-captopril GFR results are summarized in Figure 2.
Evaluation of individual renal functionDifferential renal function, estimated through pre- and post-captopril 99mTc-DMSA scintigraphies, was multiplied by the baseline and post-captopril GFR. The resultant function of each kidney was compared, considering 25 stenotic and 17 non-stenotic units. Stenotic kidneys had a lower baseline GFR than non-stenotic kidneys (18.4 ml/min vs. 32.8 ml/min; p=0.01), as well as a lower post-captopril GFR (p=0.04); however, a significant difference in the baseline/post-captopril ratio was not observed (p=0.27).
Follow-up of patients with RASTherapy strategies were determined after completion of clinical and radiological investigations, and the decision to treat RAS patients with angioplasty was mainly based on the arteriographic characteristics of the lesion. Optimization of antihypertensive therapy was the therapeutic choice in eleven cases (eight males, 62.8±6 years), and interventional procedures (angioplasty in nine cases, nephrectomy in one) were adopted in the other ten patients (four males, 61.4±11 years). No significant difference was found between those patients receiving drugs or submitted to angioplasty regarding age, time of onset of hypertension, number of antihypertensive drugs in use, or systolic blood pressure (171±22 vs. 153±20 mmHg, p=0.07), but the mean diastolic blood pressure was higher in patients submitted to angioplasty (97±12 vs. 85±14 mmHg, p=0.04).
After a mean follow-up of 379 days (range of 206–663 days), 15 patients had a good clinical response, as previously defined. Seven responders had been treated clinically (7/11=63% response rate) and eight had undergone interventions (8/10=80% response rate), with no significant difference between treatment strategies (p=0.36). There was a non-significant tendency towards a better response in patients with a higher baseline GFR (responders 53.7±22 ml/ min vs. non-responders 36.0±14 ml/min, p=0.09); however, no differences in the absolute or percentage decrease in GFR after captopril administration were observed. Considering only those 10 patients who received interventional therapy for RAS, no significant difference between responders and non-responders in either the baseline GFR or baseline/post-captopril ratio was observed (p=0.83).
DISCUSSIONUse of clinical improvement after vascular intervention as a criterion for diagnosing RVH reinforces that currently available techniques are not sufficiently accurate for this diagnosis. Captopril renal scintigraphy is a predictor of blood pressure reduction after surgery or angioplasty, 7–9 but it may be hampered by dehydration, hydronephrosis, or other factors that affect the indirect analysis of renogram curves. A method capable of directly detecting captopril-induced changes in GFR, such as the one presented here, may help to select patients who may benefit from dilation procedures.
This study showed that a one-day protocol with sequential measurements of 51Cr-EDTA clearance could be used to identify captopril-induced changes in GFR. Captopril induced a significantly greater decrease in the GFR of patients with confirmed RAS than in a control group of hypertensive patients without RAS. An exacerbated response to the inhibition of the renin-angiotensin system is a plausible explanation for this finding, although other variables, such as a lower baseline GFR, could have influenced the response.
The one-day protocol described in this study is practical for clinical use and avoids the interference of factors such as patient preparation (including hydration status and suspension of antihypertensive drugs). On the other hand, the comparison of early (two to four-hour) and delayed (six to eight-hour) samples can result in a systematic error of GFR determination, which could be avoided if baseline and post-captopril studies are performed with different injections on different days with sample collection performed at exactly the same intervals. Importantly, a systematic error does not explain the observed difference between groups of patients with and without RAS. The doses of captopril used in renal scintigraphy vary from 25 to 50 mg, leading to a peak plasma level at 40 to 60 minutes after its oral administration. We explored a period during maximum activity of the drug by using a 50 mg captopril dose and collecting plasma samples at six and eight hours (one and three hours after captopril administration).
An important limitation of 51Cr-EDTA clearance is that an in vitro technique does not permit determination of individual renal function. The global GFR of both kidneys is measured, and a decrease in filtration pressure of a stenotic kidney can be partially compensated for by an increased flow in the contralateral kidney. An experimental study confirmed that a captopril-induced decrease in inulin clearance (31%) occurs in a kidney with RAS, with no significant effect in the contralateral non-RAS kidney.12
Renal uptake of 99mTc-DMSA is strongly reduced after use of an ACE inhibitor in the presence of RVH.13 Although DMSA is well known as a tubular agent, the tracer is partially filtered in the glomerulus and is reabsorbed by tubular cells, which explains the observed effect of captopril.14 In our study, post-captopril 99mTc-DMSA scintigraphy showed no significant variation in differential function as compared to the baseline study, even in patients with RAS and with significant decreases in the global GFR. DMSA uptake mechanisms are not completely understood, and a proportion of 35% GFR to 65% direct tubular uptake has been proposed by some authors.14 DMSA uptake may be only partially or not influenced by a decrease in GFR if reabsorption of filtered radiopharmaceuticals is not the dominant mechanism in chronically ischemic or poorly functioning kidneys.
The lack of a direct comparison to captopril renography is the main limitation of this study because the functional significance of detected RAS is not clear. When the present study was designed, we believed that the clinical response to angioplasty would be the final criterion for RVH diagnosis. DTPA renal scintigraphy was performed in a non-systematic manner only when requested by the nephrologists. Captopril renography results may be influenced by different interpretation criteria15 and are usually less accurate in azotemic patients. When performed in patients with ischemic nephropathy or a poorly functioning kidney, as many as 50% of the studies suggest an indeterminate probability of RVH.16,17 Absolute GFR measurement increases renogram sensitivity, and the combined analysis of renogram and GFR resulted in a specificity of 100% for exclusion of RAS in a prospective study of thirty hypertensive patients.18
Plasma clearance of 51Cr-EDTA can be used to estimate GFR with high accuracy, and it is one of the best available indices of renal function, even for patients with a GFR as low as 15 ml/min.19 Quantitative determination of baseline and post-captopril GFR with EDTA could reduce the number of indeterminate results even in those patients with poor renal function.9 However, evaluation of atrophic/hypo-functioning kidneys may be limited by the precision of measurements (improved with in vitro 51Cr-EDTA clearance) and by the coexistence of different vascular and fluid retention mechanisms in the genesis of hypertension.
Although the worsening of renal function following captopril administration is taken as an indicator of RVH, baseline/post captopril changes in GFR ratios did not allow for discrimination of responders to revascularization procedures from non-responders, limiting the clinical implication of this study.
The small number of patients and non-randomization of therapeutic procedures do not allow for a definitive conclusion to be drawn regarding the prognostic significance of the method.
CONCLUSIONIn conclusion, the baseline and post-captopril GFR can be quantitatively measured by 51Cr-EDTA clearance in a one-day procedure, thereby allowing for avoidance of any variation in preparation or other clinical conditions.
Captopril induces a significantly greater drop in the GFR of hypertensive patients with RAS than in those without RAS, although this decrease was not related to a clinical response after intervention in this limited number of cases.