To investigate the association among hypertension, tinnitus, andsensorineural hearing loss and evaluate the influence of other covariates onthis association.
METHODS:Baseline data (2008-2010) from the Brazilian Longitudinal Study of AdultHealth (ELSA-Brasil) were analyzed. Altogether, 900 participants wereevaluated. The baseline assessment consisted of a 7-hour examination toobtain clinical and laboratory variables. Hearing was measured usingpure-tone audiometry.
RESULTS:Overall, 33.3% of the participants had hypertension. Participants withhypertension were more likely to be older, male, and diabetic compared tothose without hypertension. The prevalence of tinnitus was higher amonghypertensive participants and the odds ratio for tinnitus was higher inparticipants with hypertension than in those without hypertension. However,the difference was not significant after adjusting for age. Audiometricresults at 250-8,000 Hz were worse in participants with hypertension than inthose without hypertension in the crude analysis; however, the differenceswere not significant after adjustment for age, sex, diagnosis of diabetes,and exposure to noise. No significant difference was observed in hearingthresholds among participants having hypertension for <6 years, thosehaving hypertension for ≥6 years, and individuals withouthypertension.
CONCLUSION:Hearing thresholds were worse in participants with hypertension. However,after adjusting for age, sex, diagnosis of diabetes, and exposure to noise,no significant differences were observed between participants with andwithout hypertension. A higher prevalence of tinnitus was observed inparticipants with hypertension compared to those without hypertension, butwithout significance after adjusting for age.
Cardiovascular risk factors such as hypertension, diabetes mellitus, and dyslipidemiahave been suggested to be associated with sensorineural hearing loss (SNHL) inprevious studies (1–3). Cochlear circulatory insufficiency might be an underlyingmechanism leading to SNHL in the presence of cardiovascular risk factors that affectthe function of the inner ear. Malfunction of the strial vasculature may decreasecochlear oxygen supplementation, disrupt ionic recycling, increase free radicalproduction, and accelerate cell loss. Possibly, the basal portion of the cochlea,which is responsible for the higher frequencies, is particularly vulnerable to thisprocess (1,4–6).
Similarly, it is possible that these changes in cochlear microcirculation resultingfrom cardiovascular risk factors may be associated with SNHL and act as supportingfactors in the pathophysiology of tinnitus (7).
Age-related hearing loss affects more than 30% of the adults aged over 50 years andits prevalence roughly doubles with each decade of life (from 45% in individualsaged 60-69 years to above 80% in individuals aged above 80 years), making it thethird leading chronic health condition among aging adults (8–11).
Hearing loss (HL) impairs communication and leads to social isolation. The resultinglow self-esteem can cause depression, cognitive decline, and dementia (6,9,11,12). Thus, prevention of HL is an important public healthtarget to mitigate its adverse effects. Therefore, it is necessary to identify themodifiable risk factors for HL (6).
Several studies have been conducted, especially in high-income countries, toinvestigate the detrimental effect of hypertension and other cardiovascular riskfactors on hearing, yielding contradictory results (1,6,11,13,14). If these cardiovascular risk factors are positively associated with HL, early intervention can be beneficial for theprevention of HL and its adverse effects. Hence, it is necessary to conduct morestudies on this subject, especially in low-income and middle-income countries.
Therefore, this study aimed to investigate the association among hypertension,tinnitus, and SNHL and assess the influence of other factors such as age, sex,exposure to noise, diagnosis of diabetes, and duration of hypertension by analyzingthe data from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil).
METHODSStudy designThis ancillary cross-sectional study included 900 ELSA-Brasil participants fromSão Paulo (total ELSA-Brasil participants in São Paulo: 5,061) whowere invited to participate in the study and agreed to undergo audiometrictesting as a part of ELSA-Brasil's baseline assessment. From the originalsample (N=901), one individual was excluded due to missing data regardingantihypertensive medications, resulting in a study sample of 900participants.
Informed consent was obtained from all participants. The study was approved bythe Ethics Committee of the University Hospital of the University of SãoPaulo (n 883/09).
The design, objectives, and cohort profile of ELSA-Brasil have been published indetail in previous reports (15,16). It is a prospective cohort studyincluding 15,105 civil servants from six Brazilian cities (São Paulo, BeloHorizonte, Porto Alegre, Salvador, Rio de Janeiro, and Vitória). All activeor retired employees aged 35-74 years were eligible for inclusion in the study.The baseline assessment consisted of a 7-hour examination. The examinations wereconducted from August 2008 to December 2010. Blood samples were obtained afterovernight fasting and glucose tolerance test (75g glucose orally) and glycatedhemoglobin (HbA1c) measurements were performed (17).
Hearing examinationAfter otological inspection, an audiological assessment was conducted. Screeningacoustic immittance measurements (Madsen Otoflex 100, Natus MedicalIncorporated, CA, USA) were performed to exclude middle ear disorders. Pure-toneaudiometry was performed using air conduction at octave frequencies from250-8,000 Hz and bone conduction at 500-4,000 Hz.
Speech tests included the speech reception threshold (SRT) and speechdiscrimination score (SDS). SRT assesses an individual's ability to hearand understand standardized three-syllable words (threshold in decibel hearinglevel [dBHL]). SDS evaluates an individual's ability to hearand understand standardized one-syllable words (percentage of words correctlyidentified).
All tests were performed using a Madsen Itera II audiometer (Natus MedicalIncorporated, CA, USA) in a soundproof room (18).
Study variablesSociodemographic characteristics and medical and occupational histories wereobtained. Hypertension was defined as reported use of medications to treathypertension, systolic blood pressure ≥140 mmHg, or diastolic bloodpressure ≥90 mmHg. Diabetes was defined as medical history of diabetes,reported use of medications to treat diabetes, fasting serum glucose ≥126mg/dL, HbA1c level ≥6.5%, or glucose level ≥200 mg/dL at 2 hoursafter oral glucose tolerance test with 75g of glucose. Dyslipidemia was definedas the reported use of lipid-lowering treatment or low-density lipoproteincholesterol level ≥130 mg/dL (17,19).
Audiometric and speech test variables were compared between individuals with andwithout hypertension. The mean values were calculated for audiometricfrequencies (hearing threshold by frequency) in both ears and for SRT and SDS ofboth ears. Eventually, for individuals with absent hearing thresholds at aspecific frequency at the maximum limit of the audiometer, the maximum value ofthe audiometer plus 1 dB was considered for the calculations. This procedure wasnecessary in less than 0.2% of the hearing tests. The presence of HL was definedas hearing threshold >25 dBHL at each audiometric frequency (20). In addition, the mean values for thelow- to middle range frequencies (250-2,000 Hz) and those for the high-rangefrequencies (3000-8,000 Hz) were calculated. The tinnitus variable was alsoinvestigated, considering the individual's perception of the symptom inany ear or head.
Statistical analysisContinuous variables were expressed as mean±standard deviation and median(25th-75th percentiles) and categorical variables wereexpressed as proportions. The chi-squared test, Kruskal-Wallis test, and one-wayanalysis of variance were used as applicable. Linear regression models werebuilt using the hearing threshold values, SRT, and SDS as dependent variables toevaluate their association with hypertension. The following models wereconstructed: (A) crude, (B) adjusted for age, (C) fully adjusted (adjusted forage, sex, and diagnosis of diabetes), and (D) fully adjusted, excludingindividuals with a history of noise exposure. The Holm-Bonferroni correction wasused to adjust p-values for multiple comparisons.
Similar models were built including only the hypertensive individuals withcomplete data on age at diagnosis (N=294) to determine if the time from thediagnosis of hypertension was associated with HL. In this analysis, the cut-offtimes were set at the sample median (6 years).
The odds ratio (OR) was calculated considering the number of individuals with HLin each frequency range (low-middle or high) with and without hypertension.
All analyses were performed using the R software, version 3.1.2 (The Rfoundation, Vienna, Austria). The significance level was set at p<0.05.
RESULTSAmong the 900 participants, 300 (33.3%) had hypertension. Table 1 shows the baseline characteristics of the ELSA-Brazilstudy population. Participants with hypertension were older (55 vs.47 years), more likely to be male (54% vs. 43.8%), and diabetics(31.7% vs. 8.5%) when compared to those without hypertension.Glucose, HbA1c, systolic and diastolic blood pressure, triglycerides, and creatininelevels were significantly higher in individuals with hypertension than in thosewithout hypertension. Results of audiometric testing at 250-8,000 Hz and SRT wereworse in participants with hypertension than in those without hypertension. Thehistory of exposure to noise was similar in both the groups (approximately 39%).Notably, the prevalence of tinnitus was higher among hypertensive individuals (45.8%vs. 39.2%) (Table 1).The point estimate OR suggested a positive association between tinnitus andhypertension, but this relationship was not statistically significant afteradjusting for age (OR=1.21, confidence interval: 0.90-1.62,p=0.197).
Baseline characteristics of the study participants.
| No hypertension N=600 | Hypertension N=300 | Total N=900 | p-value | |
|---|---|---|---|---|
| Age in years (Median [P25-P75]) | 47.0 [43.0-55.0] | 55.0 [48.0-63.0] | 49.0 [44.0-58.0] | 0.000 |
| Male sex, N (%) | 263 (43.8%) | 162 (54.0%) | 425 (47.2%) | 0.005 |
| Systolic BP (mmHg) (mean±SD) | 114.4±11.7 | 132.8±17.6 | 120.5±16.4 | 0.000 |
| Diastolic BP (mmHg) (mean±SD) | 72.1±8.3 | 83.0±10.8 | 75.8±10.5 | 0.000 |
| Diabetes, N (%) | 51 (8.5%) | 95 (31.7%) | 146 (16.2%) | 0.000 |
| Fasting plasma glucose (mg/dl) (mean±SD) | 101.0±15.2 | 116.6±40.2 | 106.2±27.3 | 0.000 |
| HbA1c (%) (mean±SD) | 5.2±0.6 | 5.7±1.2 | 5.3±0.9 | 0.000 |
| Dyslipidemia, N (%) | 240 (40.0%) | 144 (48.0%) | 384 (42.7%) | 0.027 |
| HDL cholesterol (mg/dl) (mean±SD) | 52.8±12.7 | 50.0±10.7 | 51.9±12.1 | 0.001 |
| LDL cholesterol (mg/dl) (mean±SD) | 118.8±32.6 | 117.0±35.3 | 118.2±33.5 | 0.436 |
| Triglycerides (mg/dl) (median [P25-P75]) | 100.1 [73.8-144.7] | 120.7 [93.9-161.4] | 108.8 [78.5-150.2] | 0.000 |
| Serum creatinine (mg/dl) (mean±SD) | 0.9±0.2 | 1.0±0.3 | 0.9±0.2 | 0.000 |
| Tinnitus, N (%) | 235 (39.2%) | 137 (45.8%) | 372 (41.4%)a | 0.069 |
| Noise exposure, N (%) | 222 (39.9%) | 110 (39.3%) | 332 (39.7%)b | 0.933 |
| 250 Hz RE (dBHL) (mean±SD) | 12.3±9.4 | 13.5±9.7 | 12.7±9.5 | 0.066 |
| 500 Hz RE (dBHL) (mean±SD) | 11.6±9.6 | 13.5±10.0 | 12.3±9.8 | 0.007 |
| 1000 Hz RE (dBHL) (mean±SD) | 12.1±10.5 | 13.3±11.0 | 12.5±10.6 | 0.113 |
| 2000 Hz RE (dBHL) (mean±SD) | 12.5±11.6 | 15.4±13.2 | 13.4±12.3 | 0.001 |
| 3000 Hz RE (dBHL) (mean±SD) | 14.8±14.2 | 17.8±15.1 | 15.8±14.6 | 0.004 |
| 4000 Hz RE (dBHL) (mean±SD) | 18.5±16.4 | 22.5±17.4 | 19.8±16.8 | 0.001 |
| 6000 Hz RE (dBHL) (mean±SD) | 24.2±17.7 | 28.7±20.4 | 25.7±18.7 | 0.001 |
| 8000 Hz RE (dBHL) (mean±SD) | 23.1±20.1 | 28.6±22.1 | 24.9±20.9 | 0.000 |
| 250 Hz LE (dBHL) (mean±SD) | 12.8±8.1 | 14.1±10.1 | 13.2±8.9 | 0.030 |
| 500 Hz LE (dBHL) (mean±SD) | 11.2±8.0 | 13.0±10.7 | 11.8±9.0 | 0.004 |
| 1000 Hz LE (dBHL) (mean±SD) | 11.0±9.4 | 12.9±11.1 | 11.6±10.0 | 0.006 |
| 2000 Hz LE (dBHL) (mean±SD) | 12.5±12.1 | 14.9±13.4 | 13.3±12.6 | 0.006 |
| 3000 Hz LE (dBHL) (mean±SD) | 15.5±14.6 | 19.3±16.0 | 16.8±15.2 | 0.000 |
| 4000 Hz LE (dBHL) (mean±SD) | 18.9±16.5 | 23.5±18.0 | 20.4±17.1 | 0.000 |
| 6000 Hz LE (dBHL) (mean±SD) | 26.0±17.9 | 30.0±20.3 | 27.3±18.8 | 0.003 |
| 8000 Hz LE (dBHL) (mean±SD) | 25.0±20.5 | 29.4±22.3 | 26.5±21.2 | 0.003 |
| SRT RE (dBHL) (mean±SD) | 13.6±8.7 | 15.3±9.5 | 14.1±9.0 | 0.007 |
| SRT LE (dBHL) (mean±SD) | 13.4±7.5 | 15.7±10.2 | 14.2±8.6 | 0.000 |
| SDS RE (%) (mean±SD) | 96.3±5.4 | 95.7±4.8 | 96.1±5.2 | 0.166 |
| SDS LE (%) (mean±SD) | 96.1±5.4 | 95.0±7.5 | 95.7±6.2 | 0.014 |
Sixty-three individuals were excluded due to missing data regardingexposure to noise.
SD, standard deviation; BP, blood pressure; HDL, high-densitylipoprotein; LDL, low-density lipoprotein; RE, right ear; LE, leftear; SRT, speech reception threshold; SDS, speech discriminationscore, HbA1c: glycated hemoglobin, dBHL: decibel hearing level, P:percentile.
The audiometric measurements were also analyzed using linear models. Table 2 shows the beta-coefficients for theassociation between audiometric measurements and diagnosis of hypertension. In thecrude model, most of the audiometric measurements were significantly worse inparticipants with hypertension than in those without hypertension. However, thedifferences were mostly non-significant when the models were adjusted for age, sex,diagnosis of diabetes, and exposure to noise. Two (non-adjusted) significantp-values were observed for the association between thefrequencies of 6 and 8 kHz in the left ear and hypertension. However, afteradjustment for multiple comparisons, both associations were no longer significant(p=0.510 and p=0.176, respectively).
Beta-coefficients for the association between mean audiometricmeasurements and hypertension in the crude and adjusted models.
| Crude | Adjusted for age | Fully adjusted | Fully adjusted, without exposure to noise | |
|---|---|---|---|---|
| 250 Hz RE | 1.24 (−0.08 to 2.55; p=0.066) | −0.06 (−1.4 to 1.29; p=0.934) | 0.01 (−1.38 to 1.4; p=0.987) | −0.46 (−2.34 to 1.42; p=0.633) |
| 250 Hz LE | 1.36 (0.13 to 2.59; p=0.03) | 0.22 (−1.04 to 1.48; p=0.736) | 0.29 (−1.01 to 1.59; p=0.664) | 0.03 (−1.74 to 1.8; p=0.975) |
| 500 Hz RE | 1.86 (0.51 to 3.21; p=0.007) | 0.3 (−1.07 to 1.68; p=0.663) | 0.33 (−1.08 to 1.75; p=0.645) | −0.25 (−2.22 to 1.72; p=0.804) |
| 500 Hz LE | 1.84 (0.59 to 3.08; p=0.004) | 0.57 (−0.7 to 1.85; p=0.378) | 0.54 (−0.77 to 1.86; p=0.417) | −0.15 (−1.97 to 1.67; p=0.872) |
| 1000 Hz RE | 1.19 (−0.28 to 2.67; p=0.113) | −0.78 (−2.26 to 0.7; p=0.303) | −0.71 (−2.24 to 0.82; p=0.360) | −1.38 (−3.46 to 0.7; p=0.194) |
| 1000 Hz LE | 1.93 (0.55 to 3.31; p=0.006) | 0.09 (−1.3 to 1.48; p=0.902) | 0 (−1.43 to 1.44; p=0.996) | −0.29 (−2.29 to 1.72; p=0.78) |
| 2000 Hz RE | 2.9 (1.21 to 4.59; p=0.001) | −0.03 (−1.67 to 1.62; p=0.976) | −0.08 (−1.78 to 1.61; p=0.923) | −0.32 (−2.54 to 1.9; p=0.776) |
| 2000 Hz LE | 2.44 (0.7 to 4.17; p=0.006) | −0.66 (−2.35 to 1.02; p=0.443) | −0.98 (−2.71 to 0.75; p=0.267) | 0.07 (−2.27 to 2.4; p=0.956) |
| 3000 Hz RE | 2.96 (0.95 to 4.97; p=0.004) | −0.75 (−2.68 to 1.19; p=0.451) | −1.4 (−3.36 to 0.56; p=0.161) | −0.07 (−2.54 to 2.4; p=0.958) |
| 3000 Hz LE | 3.77 (1.68 to 5.87; p=0) | −0.13 (−2.14 to 1.89; p=0.9) | −0.85 (−2.88 to 1.18; p=0.412) | 0.23 (−2.36 to 2.81; p=0.863) |
| 4000 Hz RE | 3.95 (1.63 to 6.27; p=0.001) | −0.37 (−2.6 to 1.86; p=0.745) | −1.41 (−3.63 to 0.81; p=0.213) | 0.17 (−2.55 to 2.88; p=0.905) |
| 4000 Hz LE | 4.61 (2.25 to 6.96; p=0) | 0.11 (−2.14 to 2.37; p=0.922) | −0.81 (−3.06 to 1.43; p=0.478) | 0.87 (−1.84 to 3.58; p=0.529) |
| 6000 Hz RE | 4.53 (1.95 to 7.11; p=0.001) | −0.81 (−3.24 to 1.61; p=0.511) | −1.75 (−4.21 to 0.72; p=0.165) | −0.6 (−3.77 to 2.58; p=0.713) |
| 6000 Hz LE | 4.01 (1.42 to 6.61; p=0.003) | −1.66 (−4.06 to 0.75; p=0.177) | −2.65 (−5.09 to −0.2; p=0.034) | −0.54 (−3.67 to 2.58; p=0.734) |
| 8000 Hz RE | 5.43 (2.56 to 8.31; p=0) | −1.4 (−4 to 1.2; p=0.292) | −2.26 (−4.91 to 0.4; p=0.096) | −0.76 (−4.29 to 2.78; p=0.675) |
| 8000 Hz LE | 4.38 (1.45 to 7.31; p=0.003) | −2.66 (−5.3 to −0.03; p=0.048) | −3.48 (−6.17 to −0.8; p=0.011) | −1.65 (−5.22 to 1.92; p=0.365) |
Fully adjusted models were adjusted for age, sex, and the diagnosisof diabetes. Non-corrected p-values are presented in the table.
RE, right ear; LE, left ear.
A subgroup analysis of hypertensive participants was also performed to evaluate theassociation between HL and the time from the diagnosis of hypertension, comparingthese individuals with those without hypertension (Table 3).
Beta-coefficients for the association between mean audiometricmeasurements and hypertension with time from the diagnosis <6 years(N=141) and ≥6 years (n=153) in the crude and adjustedmodels.
| <6 years | Crude | Adjusted for age | Fully adjusted |
|---|---|---|---|
| 250 Hz RE | 0.61 (−1.13 to 2.35; p=0.493) | 0.09 (−1.81 to 1.63; p=0.920) | 0.01 (−1.73 to 1.74; p=0.995) |
| 250 Hz LE | 0.61 (−1.01 to 2.24; p=0.461) | 0 (−1.61 to 1.61; p=0.997) | 0.2 (−1.42 to 1.82; p=0.811) |
| 500 Hz RE | 1.13 (−0.66 to 2.92; p=0.217) | 0.29 (−1.46 to 2.05; p=0.743) | 0.31 (−1.46 to 2.09; p=0.730) |
| 500 Hz LE | 1.21 (−0.44 to 2.87; p=0.150) | 0.53 (−1.09 to 2.16; p=0.520) | 0.57 (−1.07 to 2.22; p=0.495) |
| 1000 Hz RE | 0.38 (−1.58 to 2.33; p=0.707) | −0.68 (−2.58 to 1.21; p=0.481) | −0.73 (−2.64 to 1.18; p=0.454) |
| 1000 Hz LE | 1.23 (−0.61 to 3.06; p=0.190) | 0.24 (−1.54 to 2.01; p=0.793) | 0.13 (−1.66 to 1.93; p=0.884) |
| 2000 Hz RE | 2.89 (0.65 to 5.13; p=0.012) | 1.27 (−0.82 to 3.37; p=0.234) | 1.04 (−1.07 to 3.15; p=0.334) |
| 2000 Hz LE | 2.36 (0.06 to 4.66; p=0.045) | 0.65 (−1.49 to 2.8; p=0.550) | 0.18 (−1.98 to 2.34; p=0.870) |
| 3000 Hz RE | 2.71 (0.05 to 5.37; p=0.047) | 0.66 (−1.8 to 3.13; p=0.597) | −0.28 (−2.72 to 2.15; p=0.819) |
| 3000 Hz LE | 3.07 (0.29 to 5.85; p=0.030) | 0.94 (−1.63 to 3.51; p=0.475) | −0.13 (−2.66 to 2.39; p=0.918) |
| 4000 Hz RE | 2.97 (−0.1 to 6.05; p=0.058) | 0.61 (−2.23 to 3.46; p=0.673) | −0.8 (−3.57 to 1.97; p=0.572) |
| 4000 Hz LE | 4.42 (1.3 to 7.55; p=0.006) | 1.93 (−0.94 to 4.81; p=0.188) | 0.53 (−2.26 to 3.32; p=0.709) |
| 6000 Hz RE | 3.03 (−0.38 to 6.44; p=0.082) | 0.13 (−2.96 to 3.22; p=0.936) | −1.04 (−4.11 to 2.02; p=0.505) |
| 6000 Hz LE | 2.24 (−1.19 to 5.67; p=0.201) | −0.84 (−3.91 to 2.22; p=0.589) | −2 (−5.04 to 1.05; p=0.199) |
| 8000 Hz RE | 3.37 (−0.43 to 7.17; p=0.082) | −0.34 (−3.65 to 2.97; p=0.842) | −1.39 (−4.69 to 1.91; p=0.410) |
| 8000 Hz LE | 3.23 (−0.65 to 7.11; p=0.103) | −0.64 (−3.99 to 2.72; p=0.710) | −1.71 (−5.05 to 1.64; p=0.318) |
| ≥6 years | Crude | Adjusted for age | Fully adjusted |
|---|---|---|---|
| 250 Hz RE | 2.02 (0.33 to 3.7; p=0.019) | 0.17 (−1.57 to 1.92; p=0.846) | 0.25 (−1.58 to 2.08; p=0.788) |
| 250 Hz LE | 2.15 (0.57 to 3.72; p=0.008) | 0.54 (−1.1 to 2.17; p=0.521) | 0.51 (−1.19 to 2.22; p=0.556) |
| 500 Hz RE | 2.64 (0.91 to 4.38; p=0.003) | 0.44 (−1.34 to 2.22; p=0.629) | 0.46 (−1.4 to 2.32; p=0.629) |
| 500 Hz LE | 2.5 (0.9 to 4.1; p=0.002) | 0.7 (−0.95 to 2.36; p=0.404) | 0.6 (−1.13 to 2.33; p=0.495) |
| 1000 Hz RE | 1.98 (0.09 to 3.87; p=0.041) | −0.81 (−2.73 to 1.11; p=0.41) | −0.62 (−2.63 to 1.39; p=0.544) |
| 1000 Hz LE | 2.66 (0.88 to 4.43; p=0.003) | 0.04 (−1.76 to 1.85; p=0.963) | −0.02 (−1.91 to 1.87; p=0.982) |
| 2000 Hz RE | 2.93 (0.76 to 5.1; p=0.008) | −1.35 (−3.47 to 0.78; p=0.215) | −1.36 (−3.58 to 0.86; p=0.229) |
| 2000 Hz LE | 2.5 (0.27 to 4.73; p=0.028) | −1.99 (−4.17 to 0.19; p=0.073) | −2.34 (−4.61 to −0.08; p=0.043) |
| 3000 Hz RE | 3.39 (0.81 to 5.97; p=0.01) | −2.01 (−4.51 to 0.5; p=0.117) | −2.53 (−5.09 to 0.03; p=0.053) |
| 3000 Hz LE | 4.49 (1.8 to 7.18; p=0.001) | −1.15 (−3.76 to 1.46; p=0.389) | −1.66 (−4.32 to 0.99; p=0.219) |
| 4000 Hz RE | 5.05 (2.07 to 8.02; p=0.001) | −1.19 (−4.08 to 1.7; p=0.421) | −2 (−4.91 to 0.91; p=0.178) |
| 4000 Hz LE | 5.08 (2.05 to 8.1; p=0.001) | −1.49 (−4.41 to 1.43; p=0.317) | −2.12 (−5.06 to 0.81; p=0.156) |
| 6000 Hz RE | 6.15 (2.85 to 9.45; p=0) | −1.51 (−4.65 to 1.63; p=0.345) | −2.36 (−5.58 to 0.86; p=0.151) |
| 6000 Hz LE | 5.94 (2.62 to 9.26; p=0) | −2.2 (−5.31 to 0.92; p=0.167) | −3.15 (−6.35 to 0.04; p=0.054) |
| 8000 Hz RE | 7.6 (3.92 to 11.28; p=0) | −2.19 (−5.55 to 1.17; p=0.202) | −2.93 (−6.39 to 0.54; p=0.098) |
| 8000 Hz LE | 5.65 (1.89 to 9.41; p=0.003) | −4.56 (−7.97 to −1.16; p=0.009) | −5.31 (−8.83 to −1.8; p=0.003) |
Fully adjusted models were adjusted for age, sex, and the diagnosisof diabetes. Non-corrected p-values are presented in the table.
RE, right ear; LE, left ear.
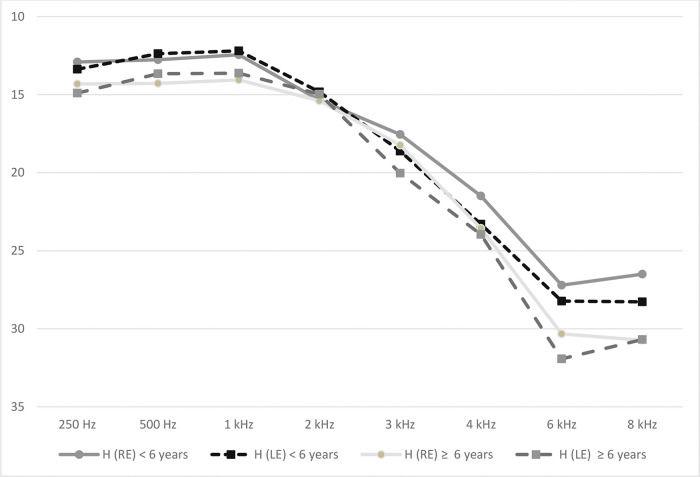
There were no significant differences in the hearing thresholds after adjustment forage or in fully adjusted models (Table 3)between individuals diagnosed with hypertension <6 years before the datacollection and individuals without hypertension. For individuals having hypertensionfor ≥6 years (time from diagnosis ≥6 years), statistically significantassociations were observed between hypertension and hearing thresholds at 2 kHz and8 kHz in the left ear in fully adjusted models. However, these associations were notsignificant after correction for multiple comparisons (p=1.000 andp=0.096, respectively). The representation of hearingthresholds by frequency for each ear of hypertensive individuals divided by the timefrom the diagnosis of hypertension is depicted in Figure 1.
Table 4 shows the number of individuals withHL in each frequency range (low-middle and high). The OR for high frequency rangeshowed a difference between individuals with and without hypertension (crude model).However, the difference disappeared in the adjusted model.
Number of individuals with hearing loss in each range of frequencies(low to middle or high) with and without hypertension, ORs, andp-values in the crude and adjusted models.
| Low- to middle-range of frequencies N (%) hearing loss | High-range of frequencies N (%) hearing loss | |
|---|---|---|
| Hypertension (N=300) | 39 (13%) | 133 (44.3%) |
| No hypertension (N=600) | 59 (9.8%) | 208 (34.6%) |
| Crude model (OR; 95% CI) | 1.39 (0.90; 2.15) | 1.51 (1.13; 2.00) |
| p-value | 0.134 | 0.004 |
| Adjusted model (OR; 95% CI) | 0.88 (0.55; 1.43) | 1.08 (0.70; 1.68) |
| p-value | 0.622 | 0.700 |
The adjusted model was adjusted for age, sex, and diagnosis ofdiabetes.
OR, odds ratio; CI, confidence interval.
Based on the baseline data from ELSA-Brasil, the association between hypertension andSNHL was investigated in 900 ELSA-Brasil participants from the São Pauloinvestigation center. In the adjusted analyses controlled for multiple risk factors,there was no association between hypertension and HL or tinnitus.
Significant differences were observed between individuals with and withouthypertension in demographic characteristics (age and sex) and in other risk factors(diabetes, glucose, HbA1c, dyslipidemia, triglycerides, and creatinine).
Altogether, 33% of the individuals from our sample had hypertension. Participantswith hypertension were more likely to be older and male when compared withindividuals without hypertension, which is consistent with the trend observed inprevious studies (1,5,8,21) as well as with the characteristics ofELSA-Brasil participants at baseline (22).
Notably, the prevalence of tinnitus was higher in participants with hypertension thanin those without hypertension (45.8% vs. 39.2%) and the OR fortinnitus was higher in hypertensive participants than in those without hypertension,although the difference was not significant after adjusting for age. A recentsystematic review on hypertension and tinnitus (7) concluded that there is an association between tinnitus andhypertension, although the relationship between the cause and the effect isuncertain. The authors also stated that changes in the cochlear microcirculationcaused by hypertension might be supporting factors in the pathophysiology oftinnitus. In the aforementioned review, the authors found five studies that assessedthe prevalence of tinnitus in patients with hypertension and the prevalence rangedfrom 7.8 to 52%.
A cross-sectional observational study by the same authors (23) found that the prevalence of hypertension in individualswith tinnitus was 44% compared to individuals without tinnitus (31.4%). The studyemphasized the association between tinnitus and hypertension, especially in olderindividuals. It is worth mentioning that the prevalence of hypertension and tinnitusincreases with age and the variation observed among different studies is influencedby the study population. Our findings suggest that there is no independentassociation between these variables.
In the crude model, pure-tone audiometry thresholds and speech test results weresignificantly worse in participants with hypertension than in those withouthypertension. These findings are consistent with the findings from previous studiesshowing that individuals with hypertension were at a high risk of HL (8,24–28). However, except those atthe frequencies of 6 and 8 kHz in the left ear, the hearing thresholds did not showsignificant differences between individuals with and without hypertension afteradjusting for age. This analysis suggested that the association between hypertensionand SNHL was mostly due to the confounding effect of age. Previous studies haveshown the influence of age on hearing thresholds (8,9,21,29,30). Indeed, age is the most important riskfactor for HL and the audiological configuration of presbycusis has the sameaudiometric characteristics as the characteristics of HL due to hypertension(bilateral and symmetrical SNHL at high frequencies) (1). Additionally, when other putative confounding variables(sex, presence of diabetes, and noise exposure) were included in the models, theassociation between hypertension and hearing thresholds was non-significant.
These findings are consistent with the findings reported by Rey et al. (31), Baraldi et al. (32), Shargorodsky et al. (33), Lin et al. (9), Oron et al.(34), and Meneses-Barriviera et al.(21), who did not find a positiveassociation between hypertension and HL. Similarly, Reed et al. (6) failed to establish a relationship betweenhypertension and HL in elderly individuals in a cross-sectional study, but found apositive association between midlife hypertension and poor hearing measured 25 yearslater.
In contrast, some studies have found an increased risk of HL in individuals withhypertension (2,13,35). Lin et al.(9) suggested that a possible explanationfor these inconsistent results is that cardiovascular risk factors are only weaklyassociated with HL and their effects might be masked by stronger risk factors suchas age, particularly in cohorts focused on older individuals.
To evaluate the association between HL and the time from the diagnosis ofhypertension, analyses were performed to categorize hypertensive individualsaccording to the time from the diagnosis, with a cut-off at the sample median (6years). However, the differences between these subgroups were not significant whenthe model was fully adjusted or after correction for multiple comparisons.
Although our results did not find a positive association between the time from thediagnosis of hypertension and HL, it is important to monitor this population toconfirm the validity of this hypothesis. Bao et al. (14) investigated the effect of blood pressure variability (BPV) onhearing. Their findings suggested that a long-term increase in BPV was associatedwith HL. The authors also emphasized that a higher BPV was more likely to lead to anunstable blood supply to the inner ear, resulting in cell death and reduced hearingsensitivity. They concluded that lowering the BPV was a novel target for preventingHL.
We used another way of data analysis, considering the audiometric results by thefrequency range. In the crude model, an increased risk of HL was observed forhigh-range frequencies in the hypertensive group than in the non-hypertensive group.However, after adjusting for age, sex, and diabetes, this difference disappeared. Asdiscussed previously, the influence of confounding variables on hearing thresholdscan be observed in this finding. In fact, chronic diseases as well as HL increasewith age and all conditions affect the blood microcirculation of the cochlea,resulting in SNHL (5).
Other aspects that contribute to the variability of findings among various studiesshould be reinforced. As an explanation for some positive results found incross-sectional studies, but not reproduced in longitudinal analyses, Shargorodskyet al. (33) suggested that this variabilitywas partly due to the differences in study designs. Other methodological aspectsdirectly affect the results and therefore, should be carefully analyzed. Manystudies have been conducted using self-reported questionnaires, which mayunderestimate the true prevalence of a disorder (8). Differences in the cut-off values, classification criteria andstudied populations (sex, race, and age) directly influence the results. Therefore,they should be considered in the analysis and comparison of the findings ofdifferent studies (8,9).
The present study found no association between hypertension and worse hearingthresholds after adjusting for age, sex, and the presence of diabetes. It should benoted that the diagnosis of hypertension was based on objective measures and thehearing thresholds were obtained through pure-tone audiometry, the gold standard foraudiological assessment. Confounding variables such as age, sex, presence ofdiabetes, noise exposure, and duration of hypertension have not been examinedsimultaneously in previous studies on the effect of hypertension on SNHL. However,the mean age of the participants and the duration of hypertension were relativelylow in our study population, which may have reduced the power of our study to detectpositive associations.
CONCLUSIONHearing thresholds were worse in participants with hypertension. However, afteradjusting for age, sex, and the presence of diabetes, no significant differenceswere found between participants with and without hypertension. A higher prevalenceof tinnitus was observed in hypertensive participants than in those withouthypertension, but the difference was not significant after adjusting for age.
AUTHOR CONTRIBUTIONSSamelli AG was responsible for the study conception and design, acquisition, analysis, and interpretation of the data, manuscript writing/editing and critical revision for important intellectual content, approval of the final manuscript version to be published, and agreement to be accountable for all aspects of the work, ensuring that the questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. Santos IS was responsible for the analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, approval of the final manuscript version to be published, and agreement to be accountable for all aspects of the work, ensuring that the questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. Padilha FYOMM, Gomes RF, Moreira RR, Rabelo CM and Matas CG were responsible for the data acquisition, manuscript editing and review, approval of the final manuscript version to be published, and agreement to be accountable for all aspects of the work, ensuring that the questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. Bensenor IM and Lotufo PA were responsible for the study conception, manuscript critical revision for important intellectual content, approval of the final manuscript version to be published, and agreement to be accountable for all aspects of the work,ensuring that the questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
This study was funded by the Ministry of Health (FINEP 01 06 0071.00), Brasília,Brazil. This research was supported by a grant from the Foundation for ResearchSupport of the State of São Paulo (FAPESP) (no. 2011/10186-9).
No potential conflict of interest was reported.