Fibromyalgia is characterized by diffuse musculoskeletal pain and discomfort. There are several reports regarding autonomic nervous system dysfunction in patients with fibromyalgia. Heart rate turbulence is expressed as ventriculophasic sinus arrhythmia and has been considered to reflect cardiac autonomic activity. Heart rate turbulence has been shown to be an independent and powerful predictor of sudden cardiac death in various cardiac abnormalities. The aim of this study is to determine whether heart rate turbulence is changed in female patients with fibromyalgia compared with healthy controls.
METHODS:Thirty-seven female patients (mean age, 40±11 years) with fibromyalgia, and 35 age- and sex-matched healthy female control subjects (mean age, 42±9 years) were included. Twenty-four hours of ambulatory electrocardiography recordings were collected for all subjects, and turbulence onset and turbulence slope values were automatically calculated.
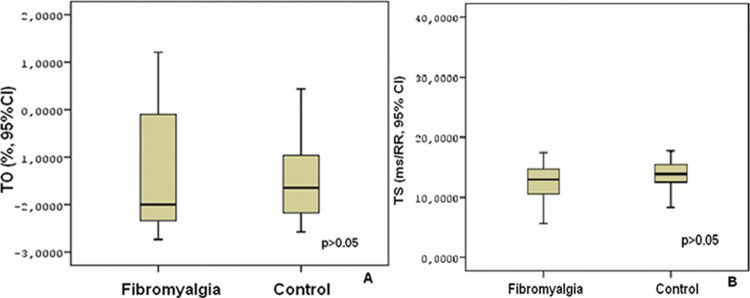
RESULTS:The baseline clinical characteristics of the two groups were similar. There were no significant differences in turbulence onset and turbulence slope measures between patients and control subjects (turbulence onset: −1.648±1.568% vs. −1.582±1.436%, p ϝ 0.853; turbulence slope: 12.933±5.693 ms/RR vs. 13.639±2.505 ms/RR, p ϝ 0.508). Although body mass index was negatively correlated with turbulence slope (r ϝ −0.258, p ϝ 0.046), no significant correlation was found between body mass index and turbulence onset (r ϝ 0.228, p ϝ 0.054).
CONCLUSION:To the best of our knowledge, this is the first study to evaluate heart rate turbulence in patients with fibromyalgia. It appears that heart rate turbulence parameters reflecting cardiac autonomic activity are not changed in female patients with fibromyalgia.
Fibromyalgia (FM) is an idiopathic disease characterized by widespread chronic pain and discomfort (1). Along with the pivotal symptom of pain, FM is accompanied by fatigue, cognitive dysfunction, mood disorder (2), nonrestorative sleep (3), and variable somatic symptoms (4). Although the etiology of the disease is not fully understood, many studies (5–7) suggest that autonomic nervous system (ANS) dysfunction plays a role in the disease process.
Heart rate turbulence (HRT), which reflects the response of the heart rate to a premature ventricular beat (PVB), is a noninvasive tool used to assess the autonomic and reflex modulations of cardiac function. HRT impairment reflects cardiac autonomic dysfunction, particularly impaired baroreflex sensitivity and reduced parasympathetic activity (8). It has been shown that HRT is an independent and powerful predictor of mortality and sudden cardiac death in various cardiac abnormalities (9). However, there is limited knowledge on HRT behavior in patients with FM. The main goal of this study was to evaluate cardiac autonomic functions in female patients with FM using HRT analysis compared with healthy subjects.
MATERIALS AND METHODSFifty-one patients with FM who were already being followed by the Department of Physical Medicine and Rehabilitation at Afyon Kocatepe University and 53 age- and sex-matched healthy controls between 1 January 2006 and 31 December 2013 were enrolled in this study. The diagnosis of FM was based on the American College of Rheumatology 1990 criteria (10).
Patients with prior myocardial infarction, hemodynamically unstable valvular heart disease, congenital heart disease, atrial fibrillation, bundle branch block, an implanted pacemaker, hypertension, diabetes mellitus, a prior cerebrovascular accident, chronic obstructive pulmonary disease, severe liver or renal insufficiency, or malignancy or patients using cardio-active drugs (especially beta blockers and/or antiarrhythmic drugs) were excluded from the study. Smokers were also excluded from both groups.
All participants' physical examinations and resting 12-lead electrocardiograms were normal. They underwent 24-hr Holter electrocardiogram monitoring. HRT could not be calculated in 14 patients with FM and in 18 controls that did not have any PVB in their Holter recordings. Therefore, 14 patients with FM and 18 control subjects were excluded from the study. HRT was calculated in 37 patients with FM and 35 control subjects who had at least one PVB in their Holter recordings. All subjects gave their informed consent prior to inclusion in the study, and all examinations were performed by the Afyon Kocatepe University Department of Cardiology.
HRT AnalysisHRT parameters, turbulence onset (TO), and turbulence slope (TS) were automatically calculated by a computer program (HRT View, Version 0.60-0.1 Software Program, Munich, Germany). Abnormal data found between 5 sinus beats before and 15 sinus beats after a PVB as well as visually observed artifacts that the program accepted as a normal PVB were excluded from the analysis. Measurements of HRT were calculated by the original method performed by Schmidt et al. (9). TO, which is a measure of the early sinus acceleration after a PVB, is expressed as a percentage and is calculated with the following formula:
[(RR1 + RR2) - (RR-2 + RR-1)] / (RR-2 + RR-1) x 100,
where RR1 and RR2 are the first and second sinus RR intervals after the PVB, and RR-1 and RR-2 are the first and the second sinus RR intervals preceding the PVB.
TS is an indicator of late sinus deceleration after PVB and is defined as the maximum positive slope of a regression line assessed over any sequence of five subsequent RR intervals within the first 20 sinus rhythm intervals after PVB (11). A TO≥0% and a TS≤2.5 msec/RR were considered abnormal. TO was separately calculated for all PVBs and then averaged, whereas TS was calculated based on an averaged local tachogram.
Statistical AnalysisStatistical analysis was performed using SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL, USA). Normally distributed continuous data were expressed as the mean±standard deviation (SD). Comparisons between independent groups were performed using Student's t test. To define the correlation between body mass index (BMI) and HRT measures, Pearson's correlation analysis was applied. A P-value <0.05 was considered statistically significant.
RESULTSThere were no significant differences between the patient and control groups with regard to age, heart rate, BMI, and systolic and diastolic blood pressures (Table 1). None of the participants presented any sustained or nonsustained ventricular tachyarrhythmia as observed with 24-h ambulatory ECG monitoring. HRT parameters, TO and TS did not significantly differ between the two groups, as shown in Figure 1.
Clinical characteristics of the patient and control groups. Data are shown as n or the mean±SD; FM-fibromyalgia.
| FM (n ϝ 37) | Control (n ϝ 35) | P-value | |
|---|---|---|---|
| Age (years) | 40±11 | 42±9 | 0.212 |
| Heart rate (/min) | 74±7 | 76±10 | 0.361 |
| Systolic blood pressure (mmHg) | 132±12 | 128±16 | 0.760 |
| Diastolic blood pressure (mmHg) | 82±10 | 78±12 | 0.250 |
| Body mass index (kg/m2) | 22.97±1.66 | 22.13±2.20 | 0.070 |
The correlation analysis revealed a weak negative correlation between BMI and TS (r ϝ -0.258, p ϝ 0.046). However, there was no significant correlation between BMI and TO (r ϝ 0.228, p ϝ 0.054) (Figure 2).
DISCUSSIONThe major finding of this study is that HRT measures of female patients with FM are not different from healthy control subjects. Autonomic dysfunction has been suggested to increase the risk for cardiovascular events and mortality (12). Although the data are not fully understood regarding the risk of cardiovascular disease in women with FM, many studies suggest ANS involvement in this disease. It is postulated that abnormal overactivity of the sympathetic system at rest and abnormal autonomic responses to sympathetic challenges could explain symptoms related to FM, such as fatigue, decreased pain threshold, anxiety, and intestinal irritability (13).
Backman et al. (5) were the first to observe striated muscle sympathetic hyperactivity in this disease. They found a lower basal muscle relaxation rate, which increased during sympathetic blockade. The authors suggested that increased muscle sympathetic nerve activity is a possible mechanism. However, Elam et al. (14) found no difference in baseline sympathetic activity between FM patients and controls when recording muscle sympathetic activity by microneurography. However, orthostatic intolerance and deranged sympathetic response to an active orthostatic stress (15–17) were detected in patients with FM.
Cardiovascular responses in patients with FM during exercise have also been examined. After a maximal treadmill test, the chronotropic reserve was found to be significantly lower in women with FM than in healthy controls. Significant reductions in heart rate recovery at 1 minute and 2 minutes after exercise (18) were also observed, suggesting an inability of the parasympathetic system to recover.
Furthermore, Martinez-Lavin et al. (19) reported that patients with FM have diminished 24-hour heart rate variability (HRV) due to an exaggerated sympathetic modulation of the sinus node. In another study (20), these authors also demonstrated that FM patients have an aberrant circadian rhythm of autonomic nervous tone, with persistent nocturnal sympathetic hyperactivity. They suggested that this may be the cause of sleep disorders in FM. These studies support the proposition that patients with FM have a decreased sympathetic response to stress.
In our study, we used HRT measurements, TO and TS to assess cardiac autonomic functions because of the controversial results of previous studies assessing cardiac autonomic activity using different methods. The heart is richly innervated by afferent and efferent vagal and sympathetic fibers and is susceptible to autonomic influences (21). Increased sympathetic and decreased vagal tone can cause arrhythmogenesis. Efferent cardiac autonomic activity is largely under the control of baroreceptor and baroreflex sensitivities (22), which are correlated with cardiac arrhythmias. HRT is an indirect cardiac autonomic function test that estimates heart rate fluctuations resulting from the stimulation of the baroreceptor arc after a single PVB. HRT is strongly correlated with spontaneous baroreceptor reflex sensitivity (BRS) and may be used instead of BRS (23). The European Society of Cardiology (24) considers HRT a marker of vagal activity and an independent indicator of total mortality. TO and TS values have strong correlations with some of the heart rate variability (HRV) parameters, including standard deviation of all normal sinus RR intervals (SDNN) and root mean squared differences of successive RR intervals (RMSSD) (25).
Most of the studies determined that HRT, baroreflex sensitivity and HRV were present in the same diseases. However, they may not always change in the same disease. For example, Bigger et al. (26) have shown that altered HRV has been observed after myocardial infarction, and baroreflex sensitivity and HRT were found to be normal in the same group. Another study (27) also showed that in the same patient group after myocardial infarction, HRV indices increased, but HRT parameters did not change. As a result, HRT and HRV indices may indicate different aspects of the ANS (26) and might provide prognostic information of incremental value.
In previous studies, HRV has been determined in patients with FM; however, HRT has not yet been investigated. Our study is the first HRT study in patients with FM. HRV was found to be decreased (19,20) in patients with FM. However, in our study, HRT did not appear to be altered in patients with FM. This may be partly due to methodological discrepancies or may possibly reveal heterogeneity in cardiac ANS in patients with FM. Constitutional and genetic factors also may play role. The physiological mechanisms underlying the various measures of HRV and HRT are different. HRV describes variations in both instantaneous heart rate and RR intervals and can reflect the coupling between the ANS and the sinoatrial node. HRT reflects the physiological biphasic response of the sinus node to PVBs, most likely because of a baroreflex arc. There is a modest correlation between HRT parameters and HRV measures.
Previous studies have demonstrated a relationship between increased BMI and FM symptoms (28,29), which may be due to physical or psychological factors, and suggested that weight loss may improve physical functioning in patients with FM. Furthermore, in one study, obesity (30) was found to be a risk factor for the development of FM. In our study, BMI values in the patient and control groups were similar. However, there are few reports evaluating the association between BMI and HRT. In one of these studies, Sarikaya et al. (31) demonstrated that TO and TS were impaired in young obese patients without comorbidities, suggesting impaired parasympathetic activity. We also found a weak negative correlation between BMI and TS; however, no significant correlation was detected between BMI and TO.
The main limitation of our study is the small sample size because a small sample size results in low statistical power for equivalency testing; thus, negative results may simply be due to chance. The lack of a reference method for studying autonomic dysfunction that could be used as a validation of the method in this study is another limitation. However, HRT is known to be a useful tool for assessing autonomic cardiac functions. Unfortunately, this type of data, collected from 24-hr Holter recordings, may not be the best for studying cardiac autonomic responses in a clinically defined group. The absence of a power spectral analysis of heart rate to a tightly controlled provocative test instead of long-term recordings obtained in circumstances that are difficult to control is another limitation of this study.
In conclusion, the HRT parameters, which determine cardiac autonomic dysfunction, did not appear to be altered in female patients with FM. We believe that comprehensive cardiac autonomic function studies with a larger number of participants should be performed in this disease.
AUTHOR CONTRIBUTIONSDursun H, Onrat E, Demirdal US, and Avsar A developed the idea for the study and helped with planning, collecting data, and writing the manuscript. Ercan E, Dundar U, Solak O, and Toktas H helped with planning, collecting data, conducting statistical analysis, and writing the manuscript.
No potential conflict of interest was reported.