Chemoreceptors play an important role in the autonomic modulation of circulatory and ventilatory responses to changes in arterial O2 and/or CO2. However, studies evaluating hemodynamic responses to hypoxia and hypercapnia in rats have shown inconsistent results. Our aim was to evaluate hemodynamic and respiratory responses to different levels of hypoxia and hypercapnia in conscious intact or carotid body-denervated rats.
METHODSMale Wistar rats were submitted to bilateral ligature of carotid body arteries (or sham-operation) and received catheters into the left femoral artery and vein. After two days, each animal was placed into a plethysmographic chamber and, after baseline measurements of respiratory parameters and arterial pressure, each animal was subjected to three levels of hypoxia (15, 10 and 6% O2) and hypercapnia (10% CO2).
RESULTSThe results indicated that 15% O2 decreased the mean arterial pressure and increased the heart rate (HR) in both intact (n = 8) and carotid body-denervated (n = 7) rats. In contrast, 10% O2 did not change the mean arterial pressure but still increased the HR in intact rats, and it decreased the mean arterial pressure and increased the heart rate in carotid body-denervated rats. Furthermore, 6% O2 increased the mean arterial pressure and decreased the HR in intact rats, but it decreased the mean arterial pressure and did not change the HR in carotid body-denervated rats. The 3 levels of hypoxia increased pulmonary ventilation in both groups, with attenuated responses in carotid body-denervated rats. Hypercapnia with 10% CO2 increased the mean arterial pressure and decreased HR similarly in both groups. Hypercapnia also increased pulmonary ventilation in both groups to the same extent.
CONCLUSIONThis study demonstrates that the hemodynamic and ventilatory responses varied according to the level of hypoxia. Nevertheless, the hemodynamic and ventilatory responses to hypercapnia did not depend on the activation of the peripheral carotid chemoreceptors.
Peripheral arterial chemoreceptors consist of aortic bodies situated in the aortic arch and carotid bifurcations (1), while central chemoreceptors are located on the ventral surface of the medulla (2). The chemoreceptors play an important role in the regulation of ventilation and circulation in response to changes in arterial O2 and/or CO2 (2-4).
It has been well established that activation of the chemoreceptors under hypoxia or hypercapnia elicits an increase in pulmonary ventilation (PV) (5-8). Nevertheless, studies in rats have shown conflicting hemodynamic responses to hypoxia. While some studies have demonstrated that hypoxia elicits a decrease in the mean arterial pressure (MAP) combined with an increase in heart rate (HR) (9-15), others have shown an increase in MAP combined with a decrease in HR (16,17).
In contrast, while there is general agreement that hypercapnia promotes a hypertensive response (18-21), there is no agreement concerning the HR response to hypercapnia. Studies have reported that bradycardia (22,23) or tachycardia (18) was elicited by hypercapnia.
Accordingly, the evident disagreement in the literature concerning the hemodynamic and ventilatory responses to hypoxia and hypercapnia requires further investigation and characterization of the role of the peripheral carotid chemoreceptors in these responses. Therefore, this study aimed to evaluate the hemodynamic and ventilatory responses to different levels of hypoxia and hypercapnia in conscious intact or carotid body-denervated (CBD) rats.
MATERIALS AND METHODSExperiments were performed on male Wistar rats (270-300 g) housed individually with free access to food and water and maintained on a 12:12-h light-dark cycle. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals [Dept. of Health, Education and Welfare, Publication No. (NIH) 85-23, Revised 1985. Office of Science and Health Reports, DRR/NIH, Bethesda, MD]. The experimental protocols used in this research were approved by the Committee of Ethics in Animal Research of the School of Medicine of Ribeirão Preto, University of São Paulo (protocol 053/2010).
Two days before the experiments, the rats were anesthetized with tribromoethanol (250 mg.kg-1, i.p., Sigma, St. Louis, MO, USA), and bilateral ligature of the carotid body arteries was performed as described elsewhere (24). Briefly, the carotid artery bifurcations were identified and isolated bilaterally. Then, the carotid body arteries were identified and tightly ligated with a suture line to block the arterial supply to the carotid bodies. The procedure was similar for rats that received the sham operation (intact), except that they did not undergo the ligature. After artery ligation, polyethylene catheters were inserted into the left femoral artery and vein for the direct measurement of arterial pressure (AP) and administration of potassium cyanide (KCN, 160 μg.kg-1, Merck, Darmstadt, Germany), respectively. Both catheters were tunneled subcutaneously and exteriorized through the back of the neck, and the surgical incision sites were closed by sutures.
For the experiments, the rats were placed into a 3.9 L Plexiglas® chamber (with sealed exit ports for catheters), which allowed them to move freely. The arterial catheter was connected to a pressure transducer (MLT0380/D, ADInstruments, Sydney, Australia), and the amplified AP signal was fed to an IBM/PC connected to a Power Lab system (ML866, ADInstruments, Sydney, Australia) and continuously sampled (2 kHz). Different gas mixtures were allowed to easily pass through the chamber to change the gas concentration. The gas concentration inside the chamber was continuously measured with an O2 analyzer (GasAlertMax XT, Canada) and a CO2 analyzer (microCapStar CO2 Monitor, PA, USA). A flowmeter gas-mixing pump (Cameron, Canada) allowed for the injection of different gas concentrations into the chamber. To measure respiratory parameters, the airflow through the chamber was interrupted for a short time (∼1 min), and the inlet and outlet ports were sealed. Pressure oscillations caused by respiratory movements were detected by a differential pressure transducer (ML141, ADInstruments, Sydney, Australia) and were digitally recorded simultaneously with AP. Volume calibration was performed during each measurement by injecting a known air volume (1 mL) into the chamber. The tidal volume (TV) was calculated offline using the formula described by Malan (25). The respiratory frequency (RF) was calculated from the extrapolation of the plethysmographic signal. Pulmonary ventilation (PV) was calculated as the product of TV and RF. MAP and HR were calculated from the arterial pulse pressure.
Experimental ProtocolEach animal was individually placed into the plethysmographic chamber at 25°C and allowed to move freely while the chamber was flushed with humidified air (21% O2 and 79% N2, 1.2 L.min-1) for 40-50 min of acclimatization. After the baseline measurements of respiratory parameters and AP, each animal was subjected to 3 levels of hypoxia (15, 10 and 6% of inhaled O2) and combined hypercapnia and hyperoxia (10% CO2+31% O2). The order of each episode of hypoxia or hypercapnia was chosen randomly, and the episodes were spaced 30 min apart.
Hypoxia was achieved by switching the gas flushed through the chamber to 100% N2. Nitrogen flow was adjusted (2, 3.5 or 5 L.min-1) to achieve the desired level of hypoxia (15, 10 or 6%) within approximately 45 s. Hypercapnia plus hyperoxia (10% CO2+30% O2) was achieved by flushing the chamber with 50% CO2 and 50% O2 (2 L.min-1) for 45 s. Each period of chemoreceptor stimulation was held for 1 minute, and the respiratory parameters, MAP and HR were measured. Subsequently, the chamber was opened to atmospheric air, and the animals were given a 30-min recovery period. At the end of the experiment, intravenous KCN was administered to verify the efficacy of carotid body denervation, as described elsewhere (17).
Statistical AnalysisThe results are expressed as means±standard errors of the mean (SEM). Changes in the MAP and PV in response to hypoxia, hypercapnia and KCN were evaluated by the paired t-test. The comparison between CBD and the intact rats was evaluated by Student's t-test. p<0.05 was considered to be statistically significant.
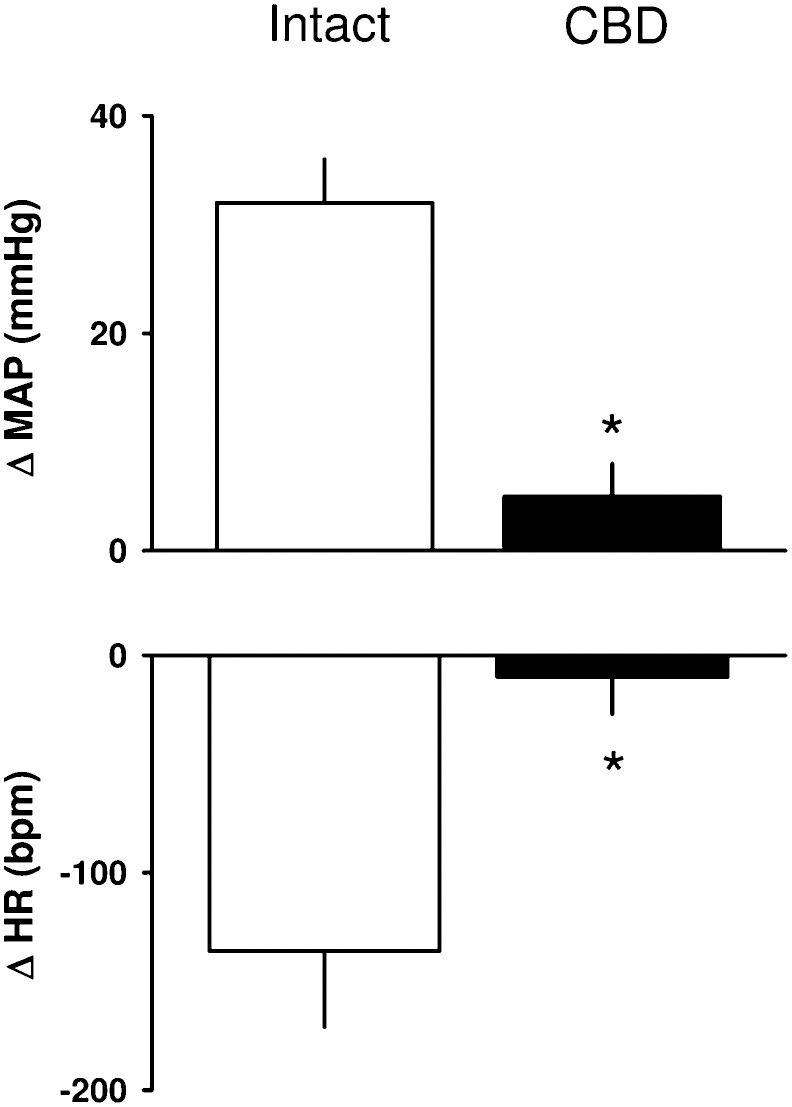
RESULTSHemodynamic responses to KCN in intact and CBD ratsThe baseline values for MAP and HR were similar between the intact (n = 8) and CBD rats (n = 7) and are shown in Table 1. KCN produced a hypertensive response combined with bradycardia in intact rats but did not change the MAP or HR in CBD rats (Figure 1.
Baseline levels of mean arterial pressure (MAP), heart rate (HR) and pulmonary ventilation (PV) in intact and carotid body-denervated (CBD) rats before chemoreceptor stimulation with KCN, hypoxia and hypercapnia. The data are expressed as means±SEM.
| INTACT | CBD | INTACT | CBD | |||
|---|---|---|---|---|---|---|
| MAP | HR | MAP | HR | PV | PV | |
| mmHg | bpm | mmHg | bpm | (mL.kg-1.min-1) | (mL.kg-1.min-1) | |
| KCN | 100±2 | 413±19 | 94±4 | 426±25 | ||
| 15% O2 | 101±4 | 411±17 | 93±5 | 415±20 | 1075±98 | 924±56 |
| 10% O2 | 103±3 | 398±10 | 99±4 | 415±20 | 1024±61 | 1012±136 |
| 6% O2 | 109±6 | 394±8 | 99±4 | 427±23 | 961±81 | 935±141 |
| 10% CO2+31% O2 | 103±4 | 401±12 | 98±4 | 439±24 | 918±105 | 983±111 |
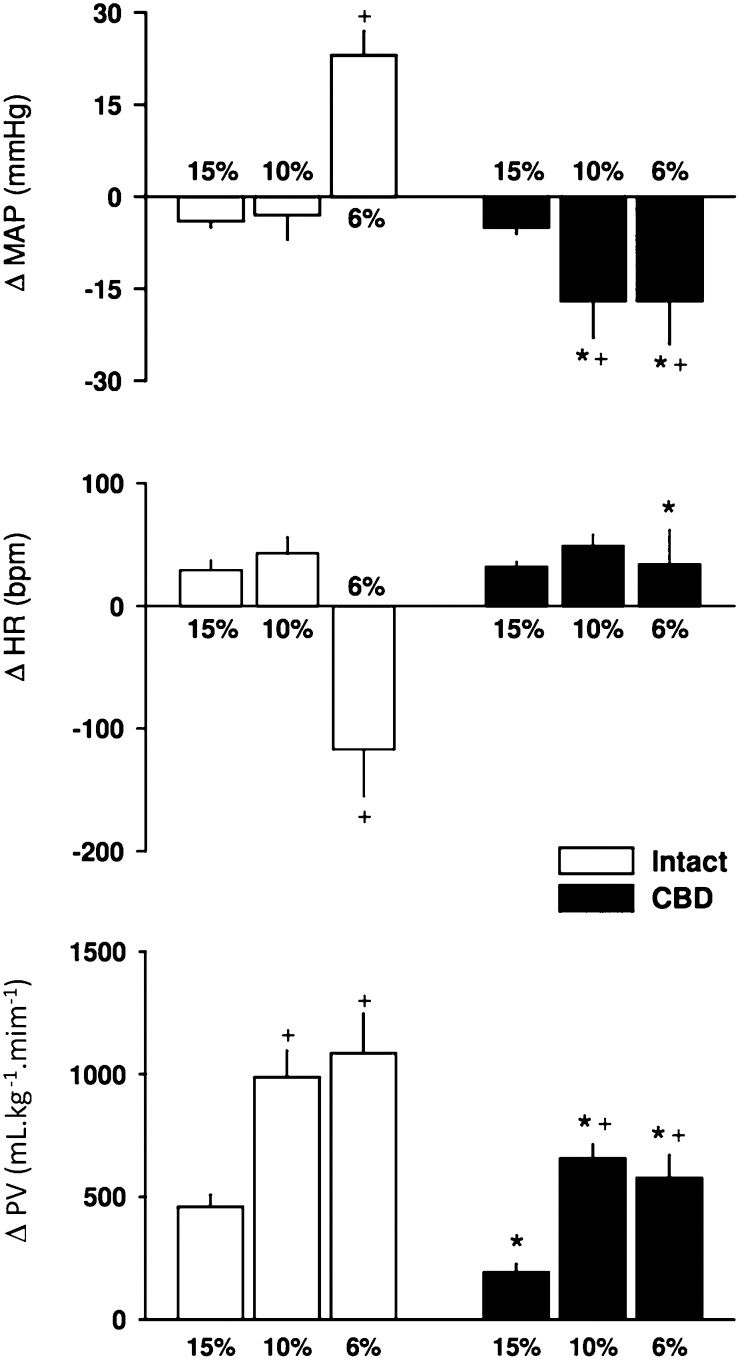
Hypoxia with 15% O2 elicited a small but significant hypotensive and tachycardic response that was similar between intact and CBD rats (Figure 2. Hypoxia with 10% O2 did not change the MAP but elicited a tachycardic response in intact rats. In CBD rats, 15% O2 elicited a marked decrease in AP and increased HR (Figure 2. The strongest hypoxia (6% O2) elicited a hypertensive and bradycardic response in intact rats and a decrease in MAP with no change in HR in CBD rats (Figure 2.
Bar graph showing the changes in the mean arterial pressure (MAP, top), heart rate (HR, middle) and pulmonary ventilation (PV, bottom) in response to 3 levels of hypoxia (15%, 10% and 6% of inhaled O2) in intact and carotid body-denervated (CBD) rats. * p<0.05 compared to 15% O2.+p<0.05 compared to the same level of hypoxia in intact rats.
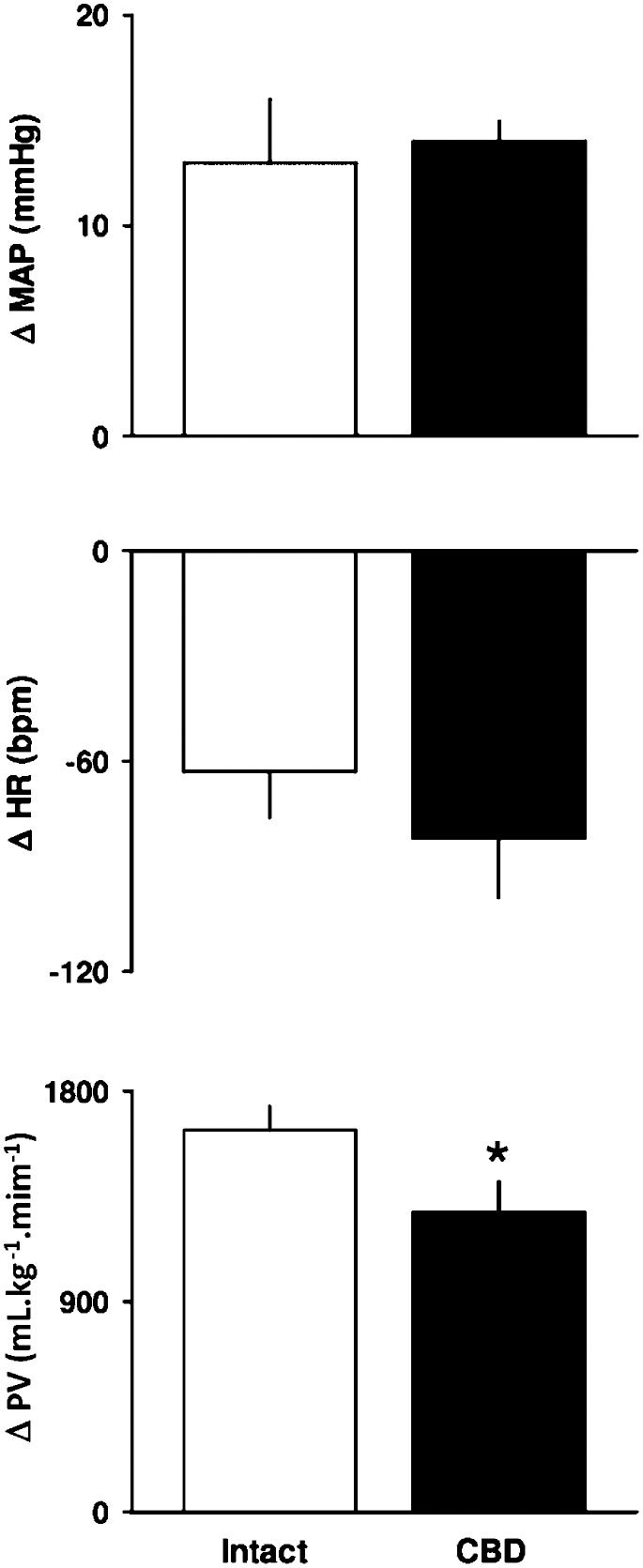
Hypercapnia (10% CO2+31% O2) promoted an increase in the MAP and a decrease in HR, which was similar in intact and CBD rats (Figure 3.
Response of pulmonary ventilation to hypoxia and hypercapniaThe baseline values for PV were similar between the intact and CBD rats (Table 1. The 3 levels of hypoxia increased PV in both groups (Figure 2. However, the PV response elicited by 15% inhaled O2 was smaller than the responses elicited by 10% or 6% O2 in both groups. Moreover, peripheral chemoreceptor denervation attenuated the ventilatory responses in CBD rats (Figures 2).
Hypercapnia (10% CO2+31% O2) promoted a similar increase in PV in both groups (Figure 3.
DISCUSSIONThe major finding of this study is that the hemodynamic response varies markedly according to the level of hypoxia (i.e., AP and HR responses can change markedly depending on the level of hypoxia applied). Moreover, this study demonstrated that the hemodynamic and ventilatory responses to the combination of hypercapnia and hyperoxia did not depend on the peripheral carotid chemoreceptors.
Hypoxia with 15% O2 produced slight but significant hypotension and tachycardia in intact and CBD rats. This finding suggests that the hemodynamic response to this level of hypoxia in rats does not depend on carotid chemoreceptor activation because a similar response was observed in CBD rats. In line with this study, Marshall and Metcalfe (10-12) also reported hypotension and tachycardia in response to 15% inhaled O2. The hypotensive response with 15% O2 might be explained by the release of adenosine from the skeletal muscle fibers (26) or blood vessel walls (27,28). Adenosine acts on endothelial A1-receptors to stimulate the synthesis and release of NO, which ultimately induces vasodilation (29). The tachycardia observed in intact and CBD rats exposed to 15% O2 might be due to the baroreflex, which is triggered by a drop in AP (30-32).
Hypoxia with 10% O2 produced a similar hemodynamic response compared to 15% O2 (i.e., hypotension and tachycardia, except that the hypotensive response did not achieve statistical significance in the intact rats). Nevertheless, the decrease in AP in response to 10% O2 in the CBD rats was markedly greater compared to 10% O2 in the intact rats. The lack of a significant hypotensive response in the intact rats might be explained by sympathetic activation elicited by the peripheral chemoreceptors to counteract a decrease in total peripheral resistance triggered by the effect of hypoxia on skeletal muscle fibers (26) or vasodilation of the blood vessel walls (27,28). The tachycardia in the intact rats could be due to the hypoxia-induced activation of the peripheral chemoreflex, which increases sympathetic activity and enhances the release of plasma catecholamines (33). Studies on conscious intact rats have shown that hypoxia with 8 or 10% O2 for 30 min decreased the MAP and increased HR (9,13,14). In addition, Sugimura et al. (34) also observed that 10% inhaled O2 for 10 min elicited hypotension combined with tachycardia in conscious rats. However, the difference between the results from previous studies (9,13,14,34) and this study might be explained by the difference in the timeframe of exposure to hypoxia among the studies (1 min vs. 10 or 30 min). The hypotensive response caused by 10% O2 in the CBD rats might be explained by attenuation of the sympathetic vasoconstrictor outflow due to the removal of the carotid chemoreceptor drive. It has been shown that hypoxia increases adrenal sympathetic nerve activity and catecholamine secretion (33), while sympathetic activation is not triggered in the absence of peripheral chemoreceptors in CBD rats. The tachycardia observed in the CBD rats exposed to 10% O2 may be due to the baroreflex, which is triggered by a fall in AP (30-32). Although the CBD rats do not have an active peripheral chemoreflex, their baroreflex is well preserved, which explains this reflex tachycardia.
Hypoxia with 6% O2 in the conscious intact rats produced marked hypertension and bradycardia, while in the CBD rats, 6% O2 produced hypotension without any change in HR. The findings of this study in conscious intact rats are in line with previous studies (16,17), indicating that maximal peripheral chemoreceptor activation is attained with 6% to 7% of inhaled O2. Previous studies in anesthetized rats have demonstrated that hypoxia promotes hypotension and tachycardia (10-12). Nevertheless, the undesirable effect of anesthesia and the longer exposure of 3 min of hypoxia might explain the discrepancy in these results (10-12) compared to this study.
Hypoxia with 6% O2 in conscious CBD rats produced significant hypotension without any change in HR. These findings were similar to the hemodynamic responses observed with 10% O2, except that the increase in baseline HR did not reach statistical significance. Thus, due to the lack of activation of the peripheral chemoreceptors in CBD rats, the hypotensive response might be explained by the direct effect of hypoxia in the blood vessel wall (27,28) and endothelial cells to promote vasodilation (28,35).
Hypercapnia (10% CO2+31% O2) increased MAP and decreased HR in intact and CBD rats. These findings indicate that the hemodynamic response to hypercapnia does not involve the peripheral carotid chemoreceptors but involves the central chemoreceptors. These results are in line with the previous findings of Walker and Brizzee (23) in conscious rats.
Ventilatory responsesHypoxia with 15, 10 and 6% O2 increased PV in the intact rats but attenuated the ventilatory response in CBD rats. These results indicate that in intact rats, PV is partially dependent on the peripheral carotid chemoreceptors because in the absence of this mechanism, the central chemoreceptors may have triggered the blunted response. It is worth mentioning that the findings concerning the PV responses observed in this study are in line with previous studies in intact and CBD rats (6,7,23). In addition, recent studies have suggested a significant role for the peripheral chemoreceptors as an important mechanism for the ventilatory response during exposure to hypercapnia (36,37).
Moreover, the response of PV to hypercapnia (10% CO2+31% O2) was similar in intact and CBD rats. Thus, the PV response to hypercapnia depends mainly on the central chemoreceptors. It is possible that the severity of hypercapnia makes the peripheral chemoreceptor less important in modulating the ventilatory response. A similar response was observed in previous studies on conscious intact rats, which indicated that hypercapnia with 6 to 7% CO2 increased PV but did not produce a significant difference in the ventilatory response compared to CBD rats (7-8).
In summary, this study shows that the hemodynamic and ventilatory responses varied according to the level of hypoxia. Nevertheless, the hemodynamic and ventilatory responses to hypercapnia (10% CO2+31% O2) did not depend on peripheral carotid chemoreceptors.
AUTHOR CONTRIBUTIONSSabino JP collected the data for this manuscript. Oliveira M and Giusti H provided technical support concerning the surgical procedures for vessel cannulation (Oliveira M) and carotid body denervation (Giusti H) and provided support during data acquisition. Glass ML provided valuable insight into data interpretation and manuscript revision. Fazan Jr R provided a substantial contribution to protocol design and data analysis. Salgado HC was the mentor of Sabino JP and was responsible for the conception of the rationale for the development of the study.
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) and Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
No potential conflict of interest was reported.