Volume replacement in septic patients improves hemodynamic stability. This effect can reduce the inflammatory response. The objective of this study was to evaluate the effect of 7.5% hypertonic saline solution versus 0.9% normal saline solution for volume replacement during an inflammatory response in endotoxemic rats.
METHODS:We measured cytokines (serum and gut), nitrite, and lipid peroxidation (TBARS) as indicators of oxidative stress in the gut. Rats were divided into four groups: control group (C) that did not receive lipopolysaccharide; lipopolysaccharide injection without treatment (LPS); lipopolysaccharide injection with saline treatment (LPS +S); and lipopolysaccharide injection with hypertonic saline treatment (LPS +H). Serum and intestine were collected. Measurements were taken at 1.5, 8, and 24 h after lipopolysaccharide administration.
RESULTS:Of the four groups, the LPS +H group had the highest survival rate. Hypertonic saline solution treatment led to lower levels of IL-6, IL-10, nitric oxide, and thiobarbituric acid reactive substances compared to 0.9% normal saline. In addition, hypertonic saline treatment resulted in a lower mortality compared to 0.9% normal saline treatment in endotoxemic rats. Volume replacement reduced levels of inflammatory mediators in the plasma and gut.
CONCLUSION:Hypertonic saline treatment reduced mortality and lowered levels of inflammatory mediators in endotoxemic rats. Hypertonic saline also has the advantage of requiring less volume replacement.
Sepsis syndrome is the leading cause of death for patients in the intensive care unit (1). The syndrome occurs as a consequence of inappropriate immune activation due to bacteria and bacterial components released during infection (2). Host receptors recognize distinct bacterial components and initiate the inflammatory response. The bacterial cell wall component lipopolysaccharide (LPS) is one of the most potent known inducers of the inflammatory response and employs Toll-like receptor 4 (TLR4) to exert its effects (3). The mechanism by which LPS induces endotoxic shock is related to its capacity to activate the NF-κB family of transcription factors, which enables the expression of several critical genes involved in the pathogenesis of septic shock: TNF-α, interleukins (IL-1β, IL-2, IL-6, and IL-8), adhesion molecules (I-CAM-1 and E-selectin), cyclooxygenase- 2, and inducible NO synthase (4). It has been shown that MAPK is functionally associated with the transcription factor NF-κB. In the absence of MAPK, the in vivo release of inflammatory mediators is down-regulated because NF-κB dependent activation is compromised (5). Hypertonic saline has been shown to reduce the activity of MAPK (6,7). The exacerbated inflammatory condition can induce cell necrosis, leading to organ dysfunction and death (2,3,8). The gut is one of the most important organs involved in sepsis, and it is the largest interface between the individual and his/her environment. An intact intestinal barrier is essential for good health and the prevention of various diseases. The intestinal barrier has various immunological and non-immunological components (4). Lung damage is a frequent cause of respiratory failure and implies the use of mechanical ventilation, which is also associated with a high risk of death in septic patients (4).
The beneficial effects of hypertonic saline were first shown by Velasco et al. in an experiment on hemorrhagic shock (9). Hypertonic saline limits local and end-organ injury in experimental pancreatitis and reduces mortality (9) by altering circulating plasma volume, reducing levels of trypsinogen, preventing acinar necrosis, and reducing levels of inflammatory cytokines and pancreatic infection. Our group showed that hypertonic saline protects the lung (10) and liver (11) from injury after pancreatitis by modulating the expression and activity of several proteins (12). Previous studies in hypovolemic shock and pancreatitis indicated that infusion of hypertonic saline reduced the production of pro-inflammatory cytokines and increased the production of anti-inflammatory cytokines, such as IL-10 (13-17). Studies using hypertonic saline in the setting of sepsis have reported hemodynamic improvements, which lead to better tissue perfusion and reduced necrosis and inflammation. A recent clinical trial using hypertonic saline (7.5% NaCl) noted an improvement in cardiac contractility and vascular tone in patients receiving hypertonic saline compared to normal saline (18). The aim of the present study was to assess the impact of hypertonic saline on the systemic inflammatory response and on the gut in experimental endotoxemia.
MATERIALS AND METHODSAll procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The study protocol was approved by the Research Ethics Committee of the University of São Paulo, School of Medicine, Hospital das Clínicas.
Endotoxemic inductionMale Wistar rats weighing 253.9±26.2 g were randomly assigned to four main groups: 1 - endotoxemic - LPS group (n = 10), induced by a single intraperitoneal injection of LPS (10 mg/kg) (extracted from Escherichia coli, serotype 026:B6; Sigma-Aldrich Canada, Ltd., Oakville, Ontario, Canada); 2 - endotoxemia treated with a single dose of hypertonic solution 7.5% (4 ml/kg) (LPS +H); 3 - group treated with a single dose of saline solution 0.9% (34 ml/kg) (LPS +S); and 4 - control group that did not receive LPS (C). The control group (C) was used to obtain baseline values. The LPS+S and LPS+H groups were treated 15 minutes after LPS injection. Samples of serum and intestine were collected 1.5, 8, and 24 h after LPS injection. The animals were anesthetized with chloral hydrate (20 mg/ml).
Experimental protocolsFor biochemical measurements, 90 rats were made endotoxemic by LPS injection, and 18 were sham. The measurements from the plasma and gut were taken 1.5, 8, and 24 h after LPS injection. Blood samples were obtained by cardiac puncture. Harvested samples from the gut (ileum) were immediately frozen in liquid nitrogen. Tissue samples were stored at −70°C until they were assayed for cytokines, as detailed below. For survival experiments, 50 animals were exposed to LPS, and their mortality was then recorded every 8 h for 24 h.
MeasurementsTissue nitriteTissue nitrate and nitrite levels were measured as an index of NO production. First, nitrate in the sample was reduced to nitrite by incubation with nitrate reductase (610 mU/mL) and NADPH (170 mM) at room temperature for 3 h. After 3 h, the nitrite concentration in the samples was measured using the Griess reaction by adding 100 mL of Griess reagent (0.1% naphthalethylenediamine dihydrochloride in water and 1% sulfanilamide in 5% concentrated H3PO4; 1:1). The optical density at 550 nm (OD 550, corrected for absorbance at 650 nm) was measured using a Spectramax microplate reader (Molecular devices, Minnesota, USA). Nitrite concentrations were calculated using an OD 550 and a standard solution of sodium nitrite prepared in phosphate-buffered saline (PBS).
MDA assayMDA formation was utilized to quantify lipid peroxidation in the tissues and was measured as thiobarbituric acid-reactive material. Tissues were homogenized (100 mg/mL) in a 1.15% KCl buffer. Two hundred microliters of the homogenate was added to a reaction mixture consisting of 1.5 mL of 0.8% thiobarbituric acid, 200 µL of 8.1% sodium dodecyl sulfate, 1.5 mL of 20% acetic acid (pH 3.5), and 600 µL of distilled water. The mixture was then heated at 90°C for 45 min. After cooling to room temperature, the samples were cleared by centrifugation (10,000 g for 10 min), and their absorbances were measured at 532 nm using 1,1,3,3-tetramethoxypropane as an external standard. The level of lipid peroxides was expressed as nmol MDA/mg protein (Bradford assay).
TNF-α, IL-6, and IL-10 — The plasma concentrations and tissue levels of immunoreactive murine TNF-α (DY410), IL-6 (DY406) and IL-10 (DY417) were determined using commercially available enzyme-linked immunoabsorbent assays (ELISA) according to the manufacturer's protocol (R&D Systems, Minneapolis, MN).
Tissue preparationFrozen tissue (100 mg) was pulverized in liquid nitrogen. Samples were homogenized in NP40 buffer containing 135 mM NaCl, 20 mM Tris (pH 8.0), 10% glycerol, and proteolytic enzyme inhibitors (40 ug/mL phenylmethylsufonylfluoride 1 mM; Sigma, St, Louis, MO). After separating the debris by centrifugation for 30 min at 10,000 rpm, the supernatants were preserved, and the protein concentrations were calculated using the Bradford method (Bio Rad, Hercules, CA). Samples were stored at –80°C until assayed.
Statistical analysisAll values were expressed as means ± standard errors of the mean (SEM). The analyses were performed using InStat Statistical Software (GraphPad, La Jolla, CA, USA). Comparisons between the experimental groups were performed using analysis of variance. A Tukey test was used as a post hoc test to compare individual groups. A p-value less than 0.05 was considered to be significant. A log-rank test was used to analyze survival.
RESULTSThe values in the sham group were the same along the time course of the study. Therefore, we pooled and used these data as time zero for the control group.
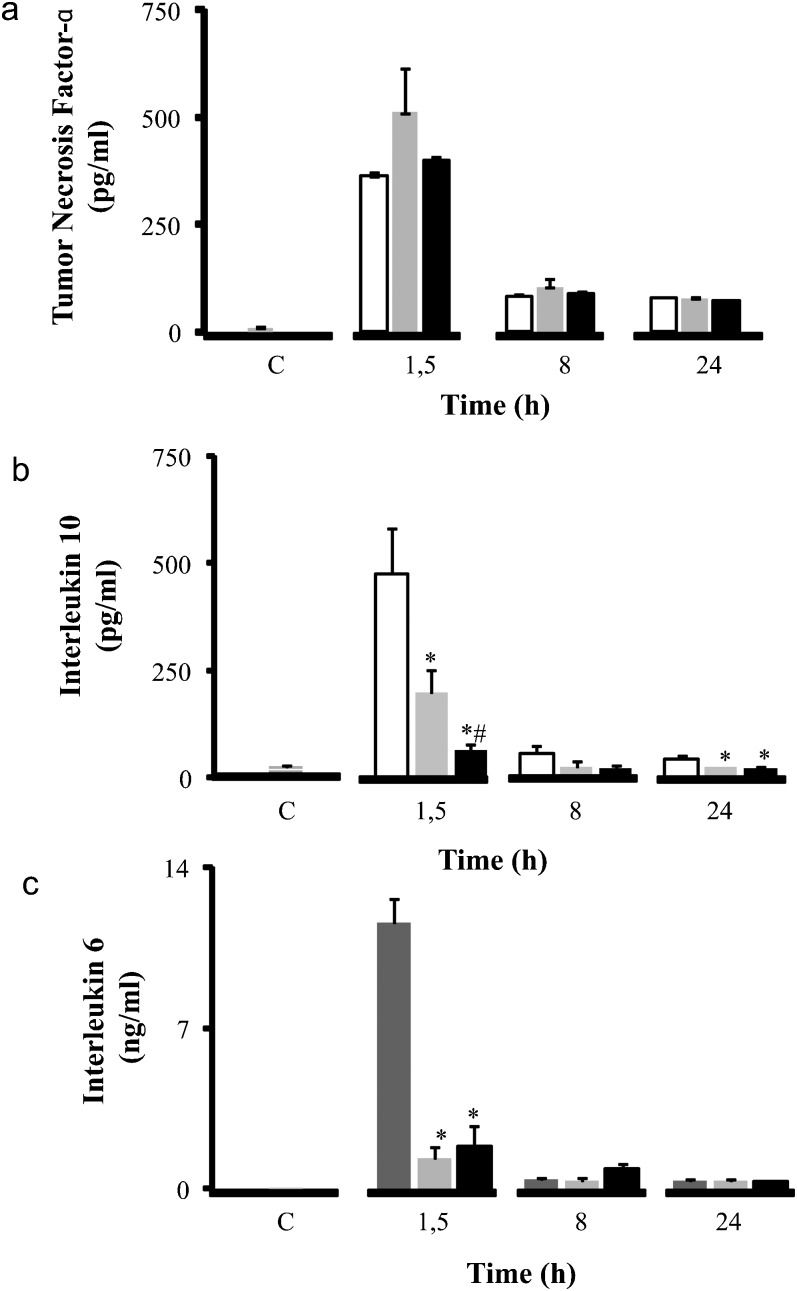
Plasma cytokine concentrations are illustrated in Figure 1. The time points chosen for this analysis (1.5, 8, and 24 h after LPS injection) correspond to the peak and relevant time course of circulating cytokine levels in this model. LPS induced an increase in plasma levels of TNF-α, IL-6, and IL-10 compared to the control group. Plasma levels of TNF-α did not change following administration of normal saline or hypertonic saline during the entire study. However, treatment with hypertonic saline did induce a significant decrease in plasma levels of the pro-inflammatory cytokine IL-6 (from 11.58±1.07 ng/ml to 1.84±0.91 ng/ml at 1.5 h), and the anti-inflammatory cytokine IL-10 (from 475.08±106.80 pg/ml to 65.89±10.31 ng/ml).
Inflammatory profile - Cytokine concentrations (pg/ml) in serum: TNF-α (a), IL-10 (b), and IL-6 (c). Wistar male rats were challenged with LPS (LPS - white) and treated with SH (LPS+H - black) and SS (LPS+S - gray). The levels of TNF- α, IL-10 and IL-6 were measured at 1.5, 8 and 24 h after the administration of LPS in serum. ∗p<0.05 when compared to the LPS group in the same period. #p<0.05 when compared to the LPS+S group in the same period. Data are means±SEM.
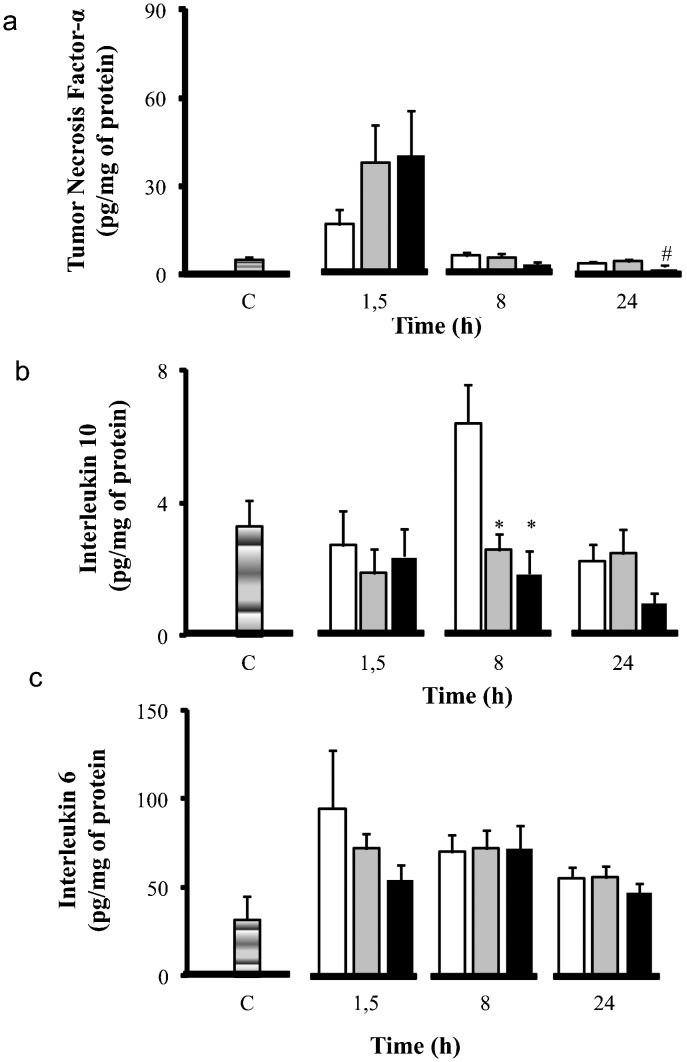
The cytokine levels in the gut are illustrated in Figure 2. LPS induced high levels of all measured cytokines compared to the control group. Volume infusion was characterized by lower tissue levels of cytokines compared to the LPS group. Hypertonic saline treatment led to an increase in TNF-α levels in the first time period measured compared to the LPS group. However, at 24 h, hypertonic saline was effective in reducing TNF-α levels compared to the LPS and the LPS+S groups (TNF-α, from 3.72±0.33 pg/mg to 2.48±0.40 pg/mg at 24 h). Hypertonic saline treatment reduced IL-6 (from 94.44±33.01 pg/mg to 53.97±8.51 pg/mg at 1.5 h) and IL-10 (from 6.57±0.76 pg/mg to 2.42±0.68 pg/mg at 8 h) levels more than the LPS group.
Inflammatory profile - Cytokine concentrations (pg/ml) in gut: TNF-α (a), IL-10 (b), and IL-6 (c). Wistar male rats were challenged with LPS (LPS - white) and treated with SH (LPS+H - black) and SS (LPS+S - gray). The levels of TNF- α, IL-10 and IL-6 were measured at 1.5, 8, and 24 h after the administration of LPS in the gut. ∗p<0.05 when compared to the LPS group in the same period. #p<0.05 when compared to the LPS+S group in the same period. Data are means±SEM.
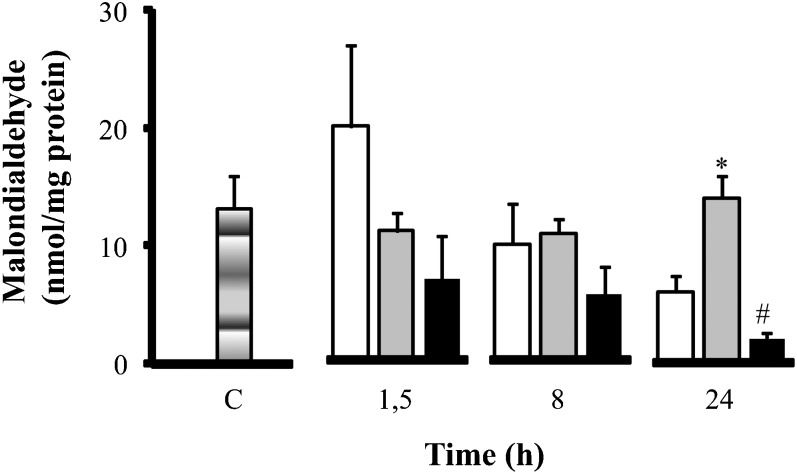
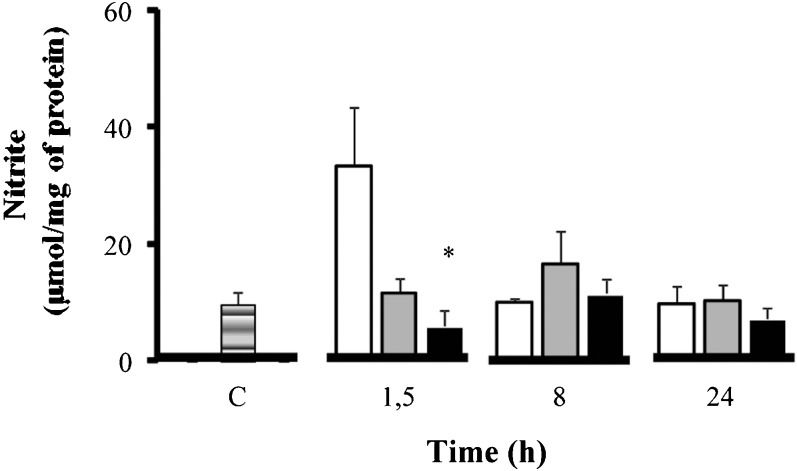
Alterations in the gut due to oxidative stress were further investigated by measuring malondialdehyde (MDA) formation (Figure 3) and nitric oxide (NOx) (Figure 4) levels. Hypertonic saline treatment reduced the levels of MDA in the gut. Hypertonic saline significantly reduced MDA levels compared to normal saline at 24 h (from 14.05±±1.40 nmol/mg to 2.12±0.48 nmol/mg at 24 h), despite having lower levels of MDA at baseline. For nitric oxide only, hypertonic saline treatment as associated with significant reduction at 1.5 h (NOx, from 33.35±9.95 µmol/mg to 7.97±2.60 µmol/ml at 1.5 h). Based on these data on MDA and nitric oxide, hypertonic saline reduced the negative impact of oxidative stress on the gut.
Oxidative stress - MDA concentrations (nmol/mg of protein) in the intestine. Wistar male rats were challenged with LPS (LPS - white); 15 minutes later, they were treated with SH (LPS+H - black) and SS (LPS+S - gray). The levels of MDA were measured in the intestine. ∗p<0.05 vs. LPS in the same period; & p<0.05 vs. LPS and HS in the same period. n = 10 mice per group. Data are means±SEM.
Oxidative stress - Nitrite concentrations (µmol/mg of protein) in the intestine. Wistar male rats were challenged with LPS (LPS - white) and treated with SH (LPS+H - black) and SS (LPS+S - gray). The levels of nitrite were measured in the intestine. ∗p<0.05 vs. LPS in the same period. n = 10 mice per group. Data are means±SEM.
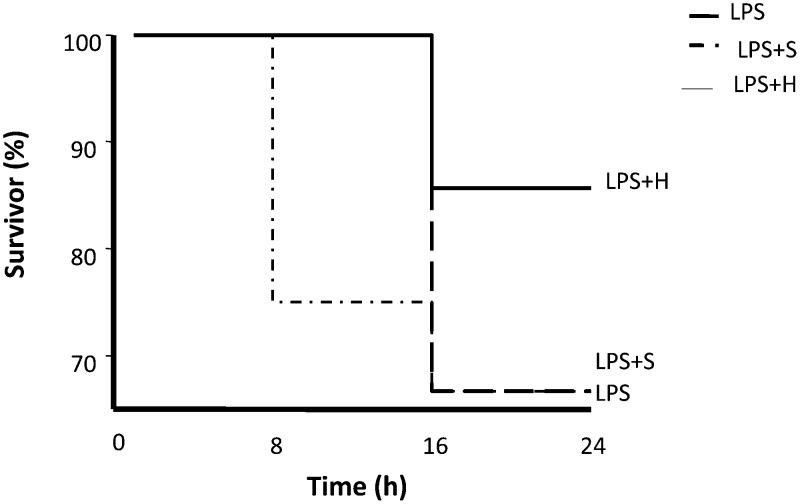
The results of the survival experiments are shown in Figure 5. Rats in the LPS group started to die at 8 h. Rats that received normal saline (Figure 5) started to die 16 h after LPS administration, with a mortality rate approaching 40% at 24 h post-LPS. The mortality rate at the end of the experiment was similar between the normal saline group and the LPS group. Rats that received hypertonic saline started to die at 16 h and had a mortality rate of 15%. The onset of death was markedly delayed in rats receiving volume treatment. Rats treated with hypertonic saline had a statistically significant survival advantage on the log-rank test.
DISCUSSIONIn this article, we investigated the effects of hypertonic saline in an experimental endotoxemic model. Hypertonic saline-treated animals had reduced levels of TNF, IL-6, IL-10, nitric oxide and superoxide in the plasma and gut. In addition, we showed that hypertonic saline treatment resulted in a higher survival rate compared to no treatment or normal saline treatment. Previous data from our laboratory and other investigators indicate that hypertonic saline has in vivo and in vitro anti-inflammatory effects. These effects are thought to be mediated, at least in part, by MAPK. It is also suggested that hypertonic saline acts mostly through cytoskeleton changes (14,19). The data presented in our study confirm and expand on these previous findings by showing that hypertonic saline has major beneficial effects in a model of endotoxemia.
Effects of hypertonic saline on the production of cytokines during endotoxemiaLPS induced a massive systemic inflammatory response, evidenced by the high levels of tissue and plasma TNF-α and IL-6. Because both TNF-α and IL-6 are proximal mediators of septic shock, it is likely that their downregulation was a central mechanism underlying the overall beneficial effects of hypertonic saline. In general, it is presumed that these mediators exert detrimental effects when extremely high levels are present. However, it has been proposed that complete inhibition of these cytokines in sepsis is detrimental because an appropriate level of cytokines is necessary for fighting bacteria (20). Hypertonic saline was able to reduce proinflammatory cytokine levels but did not completely inhibit cytokine activity. This outcome enabled an appropriate amount of physiologic cytokine activity.
Another feature of the LPS group was the high tissue and plasma levels of the anti-inflammatory cytokine IL-10. IL-10 levels were also reduced by hypertonic saline infusion. In addition, IL-10 has been shown to be crucial in preventing the overproduction of proinflammatory cytokines during systemic inflammation (21). However, this outcome did not occur in our study. Hypertonic saline decreased IL-10 and consistently reduced inflammation after LPS administration. These findings are consistent with previous studies that report the deleterious effects of increased IL-10 production in animal models of septic shock. Indeed, IL-10 was reported to suppress lymphocyte proliferation and lymphokine production during sepsis, and the administration of anti-IL-10 monoclonal antibodies has been shown to improve the survival of mice after Cecal Ligation and Puncture (CLP) (22) and in a two-hit model of EQ CLP followed by intratracheal instillation of Pseudomonas (23). The adequate levels and balance between proinflammatory and anti-inflammatory cytokines can reduce self-damage and maintain enough activity against bacteria to protect animals from exaggerated inflammation, bacterial proliferation, and invasion (20). In addition, it has been proposed that PMN activation and recruitment to various organs (especially the lung) were triggered by factors contained in mesenteric lymph tissue (24), which suggests a critical link between gut dysfunction, PMN activation, and distant organ injury in shock.
Effects of hypertonic saline on the production of nitric oxide and oxidative stress during endotoxemiaReactive oxygen species and reactive nitrogen species are produced during the inflammatory process of sepsis and endotoxemia (3,8,25). The high affinity between NO and O2- leads to the rapid production of peroxynitrite (ONOO-), which forms acidic peroxynitrous (ONOOH). Peroxynitrous is an unstable chemical species that decomposes into two free radicals: nitrite (NO2-) and hydroxyl radical (OH-) (25).
This oxidant scenario can induce important organ damage, causing dysfunction (26,27). Volume replacement reduced nitric oxide production in our study. Hypertonic saline resulted in significant nitric oxide reduction early after the onset of endotoxemia. Despite the advantages of hypertonic saline, this protective effect is not exclusive of hypertonic saline and can be related to the hemodynamic improvement produced by volume infusion. However, the data on lipid peroxidation indicated a more intense reduction with hypertonic treatment compared to normal saline, and this effect lasted for the entire study period.
Effects of hypertonic saline on survival after endotoxemiaWe found that hypertonic saline significantly improved the survival of endotoxemic rats. The effects of hypertonic saline were observed in a post-treatment strategy. Although both types of volume replacement were effective in modulating the inflammatory response, the group treated with hypertonic saline had a better oxidant profile and improved survival rate. Altogether, these data indicate that hypertonic saline may represent a valuable approach for reducing systemic inflammation, reducing oxidative stress and improving outcomes in clinical endotoxemic shock. Several distinct strategies have been previously reported to improve survival in animals challenged with endotoxemia or sepsis. The steroid hormone dehydroepiandrosterone reduced short-term mortality, an effect associated with a reduction of TNF-α release and an improvement in the activity of T cell immunity (28). Finally, neutralization of macrophage migration inhibitory factor (MIF) by anti-MIF antibodies was shown to produce a marked increase in survival, even when the treatment was delayed up to 8 h after CLP. This study defined the critical role of MIF in the pathogenesis of septic shock (29). Although it is difficult to compare the results of the aforementioned studies with our data, it is worth mentioning that hypertonic saline was effective in our severe model of LPS-induced shock. The LPS dose used in this study killed almost 35% of the control group. Our study provides evidence that hypertonic saline, beyond its immune-modulatory effects, also affords protection from several important pathophysiologic alterations associated with endotoxemia, such as oxidant stress.
This study was supported by FAPESP-09/03338-7 and 06/00443-6 and CNPQ-470744/2004-9.
No potential conflict of interest was reported.
Theobaldo MC performed experiments and measurements and analyzed results. Barbeiro HV performed experiments and measurements. Barbeiro DF performed experiments and measurements. Petroni R performed experiments and measurements. Soriano FG developed the project, analyzed results, and wrote the manuscript.