Exercise oxygen pulse (O2 pulse), a surrogate for stroke volume and arteriovenous oxygen difference, has emerged as an important variable obtained during cardiopulmonary exercise testing.
OBJECTIVESWe hypothesized that the O2 pulse curve pattern response to a maximal cycling ramp protocol exhibits a stable linear pattern in subjects reevaluated under the same clinical conditions.
METHODSWe retrospectively studied 100 adults (80 males), mean age at baseline of 59 ± 12 years, who performed two cardiopulmonary exercise testings (median interval was 15 months), for clinical and/or exercise prescription reasons. The relative O2 pulse was calculated by dividing its absolute value by body weight. Subjects were classified into quintiles of relative O2 pulse. Cardiopulmonary exercise testing results and the O2 pulse curve pattern, expressed by its slope and intercept, were compared among quintiles of relative O2 pulse at both cardiopulmonary exercise testings.
RESULTSAfter excluding the first minute of CPX (rest-exercise transition), the relative O2 pulse curve exhibited a linear increase, as demonstrated by high coefficients of determination (R2 from 0.75 to 0.90; p<0.05 for all quintiles). Even though maximum oxygen uptake and relative O2 pulse were significantly higher in the second cardiopulmonary exercise testing for each quintile of relative O2 pulse (p<0.05 for all comparisons), no differences were found when slopes and intercepts were compared between the first and second cardiopulmonary exercise testings (p>0.05 for all comparisons; except for intercept in the 5th quintile).
CONCLUSIONExcluding the rest-exercise transition, the relative O2 pulse exhibited a stable linear increase throughout maximal exercise in adults that were retested under same clinical conditions.
The stroke volume (SV) response to exercise is considered one of the most important indices of heart function.1 Unfortunately, its direct measurement during exercise requires intravascular catheterization, and therefore is rarely performed in the clinical setting. Consequently, several noninvasive methods to estimate exercise SV have been developed.2 Recently, attention has been given to the oxygen pulse (O2 pulse), a readily available variable obtained during cardiopulmonary exercise testing (CPX), calculated by the ratio of oxygen uptake (VO2) and heart rate (HR). The O2 pulse has been demonstrated to be a powerful predictor of mortality in patients with cardiovascular diseases3,4 and it has been associated to the onset of exercise-induced ischemia.5,6 Although clinically useful, the O2 pulse is not a simple variable to consider, since it is influenced by many factors that can confound its interpretation, including the presence of diastolic dysfunction,7 valvular regurgitation,8 fitness level (athletes may exhibit a plateau in oxygen pulse at higher levels of exercise, likely reflecting a physiological limitation of SV at the upper limits of HR),9 testing protocol10 and body dimensions.11
Rearranging the terms in the modified Fick equation, we have; VO2/HR = (CO × CaO2)/HR – (CO × CvO2)/HR, where CO is cardiac output and CaO2 and CvO2 are the arterial and mixed venous O2 contents, respectively. Whipp et al.11 postulated that O2 pulse, when plotted as a function of 1/HR, results in a linear relationship that extrapolates to the asymptotic O2 pulse. In other words, during progressive exercise, when VO2 changes as a linear function of HR, the O2 pulse equals the slope of the VO2-HR relationship. This relationship only holds true however, if it is assumed that the product of CO and CvO2 is constant during steady-state work rates as seen in graded exercise testing protocols (e.g.: Bruce protocol) and also that CaO2 is normally constant during exercise. To our knowledge, the O2 pulse curve pattern during the now-commonly used non-steady-state (ramp protocol) incremental exercise test has not been well described.
In addition, since SV is directly influenced by body dimensions12,13 and O2 pulse is related to the SV response to exercise, adjustments for body dimensions or weight should be included in studies aiming to evaluate the O2 pulse response to exercise. If only maximal values are considered, overweight or obese subjects would have a superior O2 pulse response, which is likely misleading considering the higher prevalence of cardiovascular disease in this particular group. This aspect has been an important limitation of both clinical6,14 and physiological studies15,16 and requires further exploration.
In the present study, we tested the hypothesis that O2 pulse corrected for body weight (hereafter termed relative O2 pulse) in response to non-steady-state incremental exercise testing demonstrates a linear pattern in a well controlled data set of subjects referred for exercise testing at our institution. In addition, we tested the hypothesis that the relative O2 pulse curve pattern during progressive exercise, expressed by its slope and intercept, remains stable during serial testing under the same clinical conditions and similar drug regimens.
MATERIALS AND METHODSStudy PopulationWe retrospectively studied a sample consisting of 502 adult non-athletes referred, at least twice, for exercise testing for clinical and/or exercise pre-participation reasons at our clinic from January 02, 2001 to October 31 2009. The study was designed to conform to the Declaration of Helsinki and approved by the Institutional Ethics Committee. Inclusion criteria consisted of patients who: a) performed two maximal cycling ramp protocol CPX at least 3 months apart; b) did not change clinical status and regular use of medications that might have affected the cardiovascular response to exercise (such as beta-blockers) at both CPXs. Subjects with valvular heart disease, lung disease, anemia and those who exhibited O2 desaturation (more than 4% at maximal effort) during exercise were excluded from the study. In addition, all CPXs stopped early for clinical indications were not considered maximal and were excluded. After applying all inclusion and exclusion criteria, 100 subjects were considered for final analyses (80 men), of whom 50% had coronary artery disease. Baseline clinical characteristics are shown in Table 1. After undergoing the first CPX, 75% of the subjects attended a supervised exercise program at our clinic at least three times a week, while the remaining 25% received advice regarding exercise.
Baseline clinical characteristics by quintiles of maximum relative O2 pulse.
| All (n = 100) | Q1 | Q2 | Q3 | Q4 | Q5 | |
|---|---|---|---|---|---|---|
| % | N/% | N/% | N/% | N/% | N/% | |
| MedicationsBeta blockers | 41 | 5/20 | 10/50 | 5/20 | 12/60*‡ | 9/45 |
| Calcium channel blockers | 16 | 4/20 | 4/20 | 4/20 | 3/15 | 1/5 |
| Nitrates | 10 | 2/10 | 2/10 | 1/5 | 4/20 | 1/5 |
| ACE-inhibitors | 14 | 2/10 | 3/15 | 7/35 | 2/10 | 0 |
| Diuretics | 12 | 6/30 | 2/10 | 3/15 | 0 | 1/5* |
| Statins | 63 | 12/60 | 16/80 | 13/65 | 13/65 | 9/45† |
| Risk factors | ||||||
| Hypertension | 43 | 8/40 | 10/50 | 9/45 | 9/45 | 7/35 |
| Dyslipidemia | 50 | 9/45 | 12/60 | 10/50 | 11/55 | 8/40 |
| Diabetes | 13 | 1/5 | 4/20 | 3/15 | 5/25 | 0 |
| Obesity (BMI ≥ 30 kg/m2) | 14 | 3/15 | 3/15 | 3/15 | 3/15 | 2/10 |
| Medical history/Procedures | ||||||
| Apparently healthy | 29 | 7/35 | 2/10 | 5/25 | 6/30 | 9/45† |
| Coronary Artery Disease | 50 | 8/40 | 13/65 | 10/50 | 12/60 | 7/35 |
| CABG | 26 | 4/20 | 9/45 | 6/30 | 5/25 | 2/10† |
| PTCA | 26 | 3/15 | 6/30 | 7/35 | 6/30 | 4/20 |
CABG, coronary artery bypass graft; PTCA, percutaneous transluminal coronary angioplasty; Q, quintile; * p < 0.05 vs. Q1; † p<0.05 vs. Q2; ‡ p<0.05 vs. Q3.
After providing written informed consent, all subjects underwent a symptom-limited CPX using an electronically-braked cycle ergometer (EC-1600; Cat Eye;, Japan or CG-04, Inbrasport, Brazil), according to an individualized ramp protocol designed to allow patients to reach maximum exercise within the desirable range of 8 to 12 minutes.17,18 The patients were verbally encouraged to exercise to volitional fatigue, regardless the maximal HR attained. No medications were stopped before the CPX. The electrocardiogram (ECG) (Cardiolife TEC 7100; Nihon-Kohden, Japan; or Elite Ergo PC 3.2.1.5; Micromed, Brazil) was continuously monitored via a single lead (CC5 or CM5). Ventilatory expired gas analysis was obtained by a metabolic system (VO2000; MedGraphics, US). The air flow and oxygen and carbon dioxide sensors were calibrated before each test using 2-liter syringes and gases with known volumes of oxygen, nitrogen, and carbon dioxide concentrations. No test results were classified as indeterminate. All exercise tests were performed, analyzed and reported according to a standardized protocol by a single experienced physician.
Hemodynamic and Ventilatory AssessmentsThe HR was analyzed beat-by-beat and expressed every 10-s. Maximum HR was considered as the highest 10-s average obtained during the CPX. The age-predicted maximum HR was also calculated by the equation [210-(0.65 × age)]. 19 Expired ventilatory data were analyzed and expressed at each 10-s. Maximum VO2 (VO2max) was expressed as the highest 60-s average value obtained during the CPX. The age-predicted VO2max was also calculated according to standard equations. 19 Delta VO2/workload was calculated as: VO2max – resting VO2 divided by maximum workload and expressed in mL.min-1.watts-1. For practical purposes, the resting VO2 while sitting on the cycle ergometer was considered to be 3.5 mL.kg-1.min-1 for all subjects. O2 pulse was calculated by dividing VO2 by HR obtained every 10-s during CPX. Maximum O2 pulse was expressed as the highest 60-s average value and was expressed in milliliters per beat. In addition, this value was expressed as a percentage of age-predicted achieved, which corresponds to the ratio between the predicted values for maximum VO2 and maximum HR. In order to remove the influence of body weight on the magnitude of O2 pulse response during CPX, its values were then divided by weight in kilograms (relative O2 pulse). In order to make the mathematical manipulations of the study easier, all results related to the relative O2 pulse were multiplied by 100.
Data management and statistical analysesOne of the strategies for testing our hypothesis was to divide the sample into quintiles, according to the results of maximum relative O2 pulse obtained during the first CPX. Dividing the sample by quintiles allowed us to compare the stability of relative O2 pulse in subjects with different fitness levels and values of maximum relative O2 pulse. Paired students t-tests were used to assess the differences for key variables between the first and second CPX, when comparisons were made for the entire sample. A χ2 statistics was used for comparisons of categorical variables among quintiles of relative O2 pulse. For comparisons made on key variables among quintiles of maximum relative O2 pulse, a repeated-measures two-way ANOVA with Greenhouse-Geisser correction 20 was performed, in which CPX (i.e., first versus second CPX) and quintiles of maximum relative O2 were the main factors. After excluding the first minute (rest-exercise transition) of the CPX, Pearson's product-moment correlations between relative O2 pulse and CPX duration were performed for each CPX, in order to test the linearity of the relative O2 pulse curve during progressive exercise. After testing the adequacy of linear regression by the magnitude of coefficient of determination of the relative O2 pulse, we then calculated the slopes and intercepts among quintiles for both CPX. To compare the slopes and intercepts, a paired student t-test was performed. All continuous data were reported as mean ± SEM or as otherwise indicated. NCSS statistical software (Kayesville, UT) was used to perform all analyses. Statistical significance was set at p<0.05 for all calculations.
RESULTSThe median time between the first and second CPX was 15 months (minimum and maximum of 5 and 62 months, respectively). Table 1 presents the baseline clinical characteristics of all patients divided by quintiles of maximum relative O2 pulse. Except for the higher proportion of apparently healthy subjects and lower proportion of coronary artery bypass surgery in the 5th quintile compared to the 2nd quintile (p<0.05), clinical characteristics were homogeneously distributed among all groups. The proportion of subjects taking beta-blockers differed only between the 4th quintile versus 1st and 3rd quintiles (p<0.05 for both comparisons).
Demographic characteristics and exercise responses for both CPX are shown in Table 2 for the entire sample. No significant differences were found for body weight (p = 0.76). Except for maximum HR (p = 0.53), diastolic blood pressure (p = 0.53) and delta VO2/workload (p = 0.14), significant differences were found for all other maximum results when the first and second CPXs were compared; the average increases were 11% and 10% for VO2max and maximum relative O2pulse, respectively.
Demographic characteristics and exercise test responses.
| VARIABLE N = 100 | First CPX | Second CPX | p-value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (yr) | 59 ± 1.2 | 60 ± 1.3 | <0.001 |
| Body mass index (kg/m2) | 26.9 ± 0.4 | 26.9 ± 0.4 | 0.82 |
| Height (cm) | 170.4 ± 0.9 | 170.5 ± 0.9 | 0.14 |
| Weight (kg) | 78.4 ± 1.3 | 78.5 ± 1.4 | 0.76 |
| Resting Values | |||
| Heart rate (beats/min) | 63 ± 1.1 | 60 ± 1.3 | 0.01 |
| Blood pressure (mm Hg) | |||
| Systolic | 133 ± 1.5 | 127 ± 2.3 | 0.02 |
| Diastolic | 77 ± 1.0 | 72 ± 1.4 | <0.001 |
| Maximum Values | |||
| Heart rate (beats/min) | 144 ± 2.6 | 144 ± 2.5 | 0.53 |
| Blood pressure (mm Hg) | |||
| Systolic | 201 ± 3.1 | 207 ± 2.6 | 0.009 |
| Diastolic | 91 ± 1.3 | 91 ± 1.3 | 0.53 |
| VO2 (mL.kg-1.min-1) | 25.1 ± 1.0 | 28.2 ± 1.0 | <0.001 |
| O2 pulse (mL.beat-1) | 13.7 ± 0.5 | 15.2 ± 0.5 | <0.001 |
| Relative O2 pulse (mL.beat-1.kg-1) | 17.4 ± 0.5 | 19.3 ± 0.5 | <0.001 |
| % achieved of age predicted O2 pulse | 110 ± 3.2 | 128 ± 3.3 | <0.001 |
| Workload (watts) | 133 ± 6.1 | 150 ± 6.7 | <0.001 |
| Delta VO2/workload (mL.min-1.watts-1) | 12.9 ± 0.22 | 13.2 ± 0.31 | 0.14 |
| Exercise duration (minutes) | 10 ± 0.2 | 11 ± 0.2 | <0.001 |
Values are mean ± SEM. VO2, oxygen uptake; O2, oxygen.
Table 3 presents the results of selected key variables divided by quintiles for maximum relative O2 pulse. No interactions between factors (quintiles and CPX) were found for any of the variables (p>0.05; Table 3). No significant differences were found for body weight, maximum HR and delta VO2/workload in each quintile (p>0.05 for all comparisons), except for body weight between first and second CPX in the 5th quintile (p<0.05). In contrast, when the first and second CPXs were compared in each quintile, significant differences were found for VO2max, maximum O2 pulse and maximum relative O2 pulse (p<0.05 for all comparisons). When the respective CPX between 1st and 5th quintiles were compared, significant differences were found for all variables (p<0.05 for all comparisons), except for body weight.
Cardiopulmonary exercise testing results by quintiles of maximum relative O2 pulse.
| Body Weight | Maximum VO2 | Maximum heart rate | Maximum O2 pulse | Maximum relative O2 pulse | Delta VO2/workload | |
|---|---|---|---|---|---|---|
| (kg) | (mL.kg-1.min-1) | (beats.min-1) | (mL.beat-1) | (mL.beat-1.kg-1)* | (mL.min-1.watts-1) | |
| Q1 (n = 20) | ||||||
| First CPX | 74.2 ± 3.6 | 16.4 ± 0.6 | 143 ± 4 | 8.6 ± 0.4 | 11.6 ± 0.3 | 12.1 ± 0.6 |
| Second CPX | 74.1 ± 3.7 | 20.2 ± 1.0 | 143 ± 4 | 10.6 ± 0.6 | 14.3 ± 0.6 | 13.3 ± 0.7 |
| Q2 (n = 20) | ||||||
| First CPX | 75.2 ± 3.3 | 19.5 ± 1.0 | 138 ± 7 | 10.8 ± 0.5 | 14.3 ± 0.1 | 12.5 ± 0.4 |
| Second CPX | 74.9 ± 3.6 | 24.3 ± 1.3 | 140 ± 6 | 13.2 ± 0.8 | 17.6 ± 0.7 | 12.9 ± 0.5 |
| Q3 (n = 20) | ||||||
| First CPX | 82.3 ± 2.7 | 22.9 ± 0.7 | 142 ± 6 | 13.4 ± 0.4 | 16.3 ± 0.2 | 13.0 ± 0.4 |
| Second CPX | 81.5 ± 3.0 | 26.4 ± 1.3 | 143 ± 5 | 15.1 ± 0.6 | 18.7 ± 0.7 | 12.7 ± 0.4 |
| Q4 (n = 20) | ||||||
| First CPX | 82.2 ± 2.6 | 27.1 ± 1.5 | 139 ± 6 | 16.2 ± 0.6 | 19.7 ± 0.2 | 12.8 ± 0.4 |
| Second CPX | 82.6 ± 5.3 | 29.6 ± 2.2 | 139 ± 6 | 17.8 ± 1.1 | 21.4 ± 1.1 | 12.6 ± 0.4 |
| Q5 (n = 20) | ||||||
| First CPX | 78.2 ± 2.4 | 39.9 ± 2.5 | 159 ± 5 | 19.5 ± 1.0 | 24.9 ± 1.0 | 13.9 ± 0.5 |
| Second CPX | 79.6 ± 2.5 | 40.3 ± 2.6 | 160 ± 6 | 19.5 ± 1.1 | 24.4 ± 1.2 | 14.8 ± 1.1 |
| p value | ||||||
| Quintile factor | 0.018 | <0.001 | 0.002 | <0.001 | <0.001 | 0.021 |
| CPX factor | 0.949 | 0.004 | <0.843 | 0.002 | <0.001 | 0.312 |
| Interaction | 0.998 | 0.740 | 0.999 | 0.601 | 0.113 | 0.687 |
Values are mean ± SEM. VO2, oxygen uptake; O2, oxygen; Q, quintile. * All results of maximum relative O2 pulse were multiplied by 100.
VO2 increased in a linear manner relative to HR in both CPX from the second minute to maximum exercise (average R2 = 0.84 for both CPX in the entire sample). The average R2 between VO2 and HR from the onset to the second minute of exercise in both CPX was 0.53. The results of the linear regression between relative O2 pulse and CPX time divided by quintiles of maximum relative O2 pulse are shown in Table 4. The high coefficient of determination (R2) in each quintile reveals the linearity of relative O2 pulse, after exclusion of the first minute of CPX. After a median time of 15 months, no significant differences were found for the slopes and intercepts between the first and second CPX in each quintile of maximum relative O2 pulse (p>0.05 for all comparisons; except for intercept comparison in the 5th quintile, p = 0.007). When extremes of quintiles for maximum relative O2 pulse (i.e., 1st versus 5th quintiles) were compared, its respective slopes were significantly different (p<0.05). In a subset analysis, similar results were found when the slopes between the first and second CPX were compared separately in men (p = 0.75) and women (p = 0.24); among subjects taking beta-blockers (p = 0.78) and in subjects with known coronary artery disease (p = 0.31).
Linear regression results by quintiles of maximum relative O2 pulse.
| R2 | Slope* (95% CI) | Slope p-value versus Q5 | Slope p-value 1st vs 2nd CPX | Intercept (95% CI) | Intercept p-value 1st vs 2nd CPX | |
|---|---|---|---|---|---|---|
| Q1 | ||||||
| First CPX | 0.78 | 0.61 (0.47 - 0.76) | <0.001 | 0.57 | 6.9 (5.8 - 8.0) | 0.22 |
| Second CPX | 0.79 | 0.68 (0.50 - 0.85) | 0.04 | 8.1 (9.1 - 12.3) | ||
| Q2 | ||||||
| First CPX | 0.75 | 0.62 (0.43 - 0.80) | 0.003 | 0.37 | 9.4 (8.3 - 10.5) | 0.13 |
| Second CPX | 0.78 | 0.73 (0.56 - 0.90) | 0.34 | 10.7 (9.11 - 14.0) | ||
| Q3 | ||||||
| First CPX | 0.82 | 0.68 (0.51 - 0.85) | 0.009 | 0.27 | 10.3 (9.1 - 11.5) | 0.82 |
| Second CPX | 0.86 | 0.79 (0.63 - 0.95) | 0.61 | 10.5 (8.7 - 12.4) | ||
| Q4 | ||||||
| First CPX | 0.84 | 0.88 (0.70 - 1.05) | 0.36 | 0.54 | 11.1 (9.7 - 12.5) | 0.10 |
| Second CPX | 0.85 | 0.81 (0.62 - 1.01) | 0.79 | 12.6 (11.0 - 14.2) | ||
| Q5 | ||||||
| First CPX | 0.90 | 0.99 (0.82 - 1.15) | - | 0.12 | 12.8 (10.9 - 14.6) | 0.007 |
| Second CPX | 0.89 | 0.85 (0.66 - 1.03) | - | 15.4 (13.6 - 17.1) |
R2, coefficient of determination for correlation between Relative O2 Pulse and CPX duration; CI, Confidence interval; Q, quintile. * Slopes significantly different from zero (p<0.05).
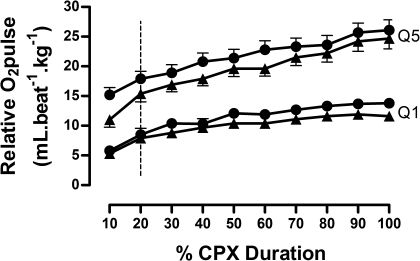
Figure 1 shows the relative O2 pulse curves as a function of percentage time during first and second CPX for 1st and 5th quintiles. No differences were found between CPXs for each quintile (p>0.05). The vertical line highlights the linearity of the curves after exclusion of the first 10% of the CPX time.
First and second quintiles of relative O2 pulse during CPX. Triangles (▴) stand for results obtained in the first CPX. Closed circles (•) stand for results obtained in the second CPX. The vertical line highlights the linearity of the curves after exclusion of the first 10% of the CPX time. All results of relative O2 pulse were multiplied by 100. The exercise responses and slopes for each quintile are shown in tables 3 and 4. Q, quintiles; CPX, cardiopulmonary exercise testing. No significant differences were found when both CPX in each quintile were compared (p>0.05 for all comparisons).
The results of the present study add to the existing body of research showing a linear increase in O2 pulse throughout maximal incremental non-steady-state exercise testing. In addition, to our knowledge, this is the first study to demonstrate the stability of the O2 pulse curve pattern in a large group of subjects under identical clinical status, who served as their own control in a test-retest design.
Our results are in agreement with previous studies, 3,5,10,21in that VO2max and maximum O2 pulse were within normal limits and were significantly higher in the second CPX (p<0.05; Table 2 and 3). Even though the purpose of our study was not to assess the influence of exercise training, the higher values observed for VO2max and maximum O2 pulse between tests probably occurred because of the training influence, since almost all the subjects increased their physical activity levels. In addition, an effect due to counseling may have occurred since the remaining 25% of the patients were underwent activity counseling after undergoing their initial CPX.
A strong debate regarding the behavior of the SV response to progressive maximum exercise still exists. 9,22–24 Variations in these studies include a decrease, 25 a plateau 26 or an increase in SV when approaching volitional exhaustion 27 when untrained, moderately trained or heart disease patients are considered. 24 The prospective design and complexity of methods for measuring SV in most studies has generally limited sample sizes, and thus limited the external validity of the results. In addition, different testing protocols (graded or constant), type of ergometer (treadmill or cycle), criteria for termination of the test (exhaustion or pre-determined % of age-predicted maximum HR) and also the lack of control of confounding variables such as body weight have limited the interpretation of previous results. Our results support the concept that SV, estimated by relative the O2 pulse response to maximum exercise, increases in a linear fashion throughout exercise in non-athletes as shown by the high R2 (Table 4). A decrease or a plateau in relative O2 pulse would lead to a reduced R2 which was not the case in our results. Our approach was novel in that we excluded the first minute of CPX to calculate the linearity and slopes of relative O2 pulse. At the onset of a ramp protocol, the lack of linearity in the intensity increment affects the linear increase of HR as a function of VO2 (average R2 = 0.53 for both CPX). 28 As a consequence, an artificial upward shift in O2 pulse slope occurs at the onset of exercise, as seen in Figure 1 during the first 10% of the test (the slopes are visually different before and after the vertical line). Thus, excluding the first minute of the CPX has an advantage in that it allows the direct use of the O2 pulse slope, obviating the need to calculate the VO2 and HR slopes. Although linearity was present irrespective of quintile of maximum relative O2 pulse (high R2 in all quintiles) it is clear that the higher the slope of the relative O2 pulse, the higher will be its linearity, as demonstrated by the positive trend shown in the results of R2 among quintiles of relative O2 pulse (Table 4).
Data have recently emerged in regard to the association between the O2 pulse pattern during CPX and the presence of ischemia during exercise 5,6,29. Belardinelli et al. 5 studied 202 patients with known coronary heart disease who underwent both myocardial scintigraphy and cycle CPX. By logistic regression analysis, the only independent predictors of a positive myocardial scintigraphy were O2 pulse flattening duration (calculated from the inflection point occurring in VO2 as related to work) and the slope of VO2/workload. The slope of VO2/workload was within normal limits from the start of exercise to a point corresponding to the onset of myocardial ischemia. However, as work rate increased further, an inflection point was evident in most patients with detectable myocardial ischemia, with the cutoff of 3.9 mL.min-1.watts-1 being the strongest independent predictor according to a hierarchical model. Supporting these results, Chaudhry et al., 29 showed that at the onset of myocardial ischemia, a decrease in the O2 pulse with increasing work rate and a abrupt decrease in the slope of VO2/workload occurred in a 68 year old woman referred for CPX as part of a preoperative evaluation. The O2 pulse patterns observed in these studies are most likely explained by reduced stroke volume at higher intensity exercise due to myocardial ischemia. In contrast, this was not the case in our relatively normal subjects, since delta VO2/workload was within normal limits in all quintiles of maximum relative O2 pulse (Table 3) and the slopes were significantly different from zero (Table 4).
A novel finding shown by the present study was that, after a median time of 15 months in between first and second CPXs, the relative O2 pulse curve pattern remained unchanged as demonstrated by the lack of significant differences in slopes and intercepts regardless the maximum relative O2 pulse presented in each quintile (Table 4). The slopes and intercepts were similar despite the significantly higher values of VO2max in second CPX in each quintile of maximum relative O2 pulse (on average 1% to 20% higher on the second CPX). This may have occurred because of the lower sub-maximal HR values during the second CPX, as a result of the improved VO2max (possible training or counseling effect). In fact, three possible combinations of VO2 and HR kinetics may occur after a period of exercise training, the first being that only the VO2 kinetics is modified with training, the second being that only the HR kinetics is modified with training and the third being that both VO2 and HR kinetics are modified with training. The first two cases imply that modifications of O2 pulse slope only mirrors the modification of VO2 or HR kinetics; if such is the case, the relevance of the O2 pulse kinetics is limited. On the other hand, as may have occurred in our study (Tables 3 and 4); when both VO2 and HR kinetics are modified, the O2 pulse kinetics do not correlate highly with any of these two variables. The results for the O2 pulse slopes were similar when comparisons between the first and second CPXs were performed, and were also similar when subsets of subjects were analyzed with known coronary artery disease and those taking beta-blockers, which extend the clinical applications of our results to these subgroups.
Finally, our study adjusted O2 pulse by body weight. Given the close relationship between SV and body dimensions, 12,13 consideration of body dimensions is necessary when evaluating the O2 pulse pattern to exercise. Otherwise, an obese subject might misleadingly have a superior O2 pulse response when compared, for example, to a lean marathoner. To our knowledge, few studies have taken into account the influence of weight on O2 pulse responses to exercise. 30-32 This aspect has been an important limitation of both clinical 6,14 and physiological studies. 15,16 In a study by Munhoz et al.,6 87 patients underwent both myocardial scintigraphy and treadmill CPX in order to compare the O2 pulse response to incremental exercise in patients with and without ischemia as detected by myocardial scintigraphy. Although a flattening of the O2 pulse response occurred in patients with extensive myocardial ischemia when compared to those with mild ischemia, the authors concluded that O2 pulse responses during exercise were not able to discriminate those with and without myocardial ischemia. Caution is in order, however, when interpreting these results, since there were significant differences in the weight of the subjects, which was heavier in those with ischemic responses. It is possible then, that patients with ischemic responses performed better in terms of O2 pulse simply because they were heavier. Unfortunately, the authors did not provide information on the relative O2 pulse responses, which limits comparisons between subjects with different body weights. The sub-maximal O2 pulse has also been reported to be similar between trained and untrained men, when trained men were on average 14 kg lighter than untrained men.16 Similarly, O2 pulse was not significantly different between obese and leaner women when obese women were 18 kg heavier on average than leaner women.15
Some limitations are of note in the present study. Even though direct measurements of SV were not made, collectively, the evidence is convincing that O2 pulse correlates well with direct measurements of SV. 11,16,21,33,34 According to the modified Fick equation, O2 pulse equals the product of SV and arterio-venous oxygen difference. Since the assessment of arterio-venous oxygen difference requires the placement of invasive catheters, we assumed that arterio-venous oxygen difference increases in a predictable way with respect to workload, reaching an approximate constant peak value at close to maximal intensity.25,35 Therefore, after the point where arterio-venous oxygen difference tends to reach its maximum value, any further increase in O2 pulse will reflect changes in SV. Finally, although the aim of the present study was not to assess the influence of exercise training of key dependent variables, we cannot exclude the possible influence of the supervised exercise program or counseling on our results. Considering all the above, some caution should be made when interpreting the results of the present study.
Clinical implicationsThe novelty of our study lays in the fact that it was the first study to demonstrate the stability of O2 pulse. By showing the O2 pulse curve stability after a median time of 15 months in subjects under similar clinical conditions and drug regimens, we reject the hypothesis that factors such as measurement variability inherent to any test (in our case CPX), could affect the O2 pulse pattern. This increases in importance considering the established association between O2 pulse curve pattern and myocardial ischemia. In other words, if such variability in the O2 pulse curve pattern was present, rejecting our hypothesis, it could be difficult to discriminate those with a flat O2 pulse curve truly caused by myocardial ischemia from those with a flat curve caused just by variations in measurements inherent to CPX.
CONCLUSIONSAfter excluding the first minute of CPX (rest-exercise transition), the relative O2 pulse exhibited a linear increase throughout maximum exercise. In addition, in a test-retest design, where subjects served as their own controls, the pattern of relative O2 pulse remained stable.
Ricardo Oliveira was supported by FAPERJ (Brazil). Claudio Gil Araújo is a recipient of research fellowships from CAPES and FAPERJ (Brazil)