Melatonin, a hormone released by the pineal gland, demonstrates several effects on the cardiovascular system. Herein, we performed a systematic review and meta-analysis to verify the effects of melatonin in an experimental model of myocardial infarction. We performed a systematic review according to PRISMA recommendations and reviewed MEDLINE, Embase, and Cochrane databases. Only articles in English were considered. A systematic review of the literature published between November 2008 and June 2019 was performed. The meta-analysis was conducted using the RevMan 5.3 program provided by the Cochrane Collaboration. In total, 858 articles were identified, of which 13 were included in this review. The main results of this study revealed that melatonin benefits the cardiovascular system by reducing infarct size, improving cardiac function according to echocardiographic and hemodynamic analyses, affords antioxidant effects, improves the rate of apoptosis, decreases lactate dehydrogenase activity, enhances biometric analyses, and improves protein levels, as analyzed by western blotting and quantitative PCR. In the meta-analysis, we observed a statistically significant decrease in infarct size (mean difference [MD], -20.37 [-23.56, -17.18]), no statistical difference in systolic pressure (MD, -1.75 [-5.47, 1.97]), a statistically significant decrease in lactate dehydrogenase in animals in the melatonin group (MD, -4.61 [-6.83, -2.40]), and a statistically significant improvement in the cardiac ejection fraction (MD, -8.12 [-9.56, -6.69]). On analyzing potential bias, we observed that most studies presented a low risk of bias; two parameters were not included in the analysis, and one parameter had a high risk of bias. Melatonin exerts several effects on the cardiovascular system and could be a useful therapeutic target to combat various cardiovascular diseases.
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone produced by the pineal gland exclusively at night and is released into the bloodstream and cerebrospinal fluid in a circadian manner to regulate several physiological and neuroendocrine functions (1-3). The effects of melatonin are dependent on non-receptor- and receptor-mediated mechanisms of action. Membrane melatonin receptors (MT1, MTNR1A, MT1, and MTRN1B) are G-protein-coupled receptors, signaling through Gi-G0 or Gq-G11 transduction pathways, depending on the target organ. Melatonin secreted at night might interact with its effector and produce immediate effects when melatonin is present in the circulation (e.g., nighttime blood pressure dipping). Moreover, during the night and through several mechanisms of action, melatonin primes prospective effects (such as controlling autonomic nervous system activity) that can be observed only during the day when no pineal melatonin production occurs (3-6).
Over the last 20 years, several studies have suggested that melatonin influences the cardiovascular system (7,8). Melatonin may have significant anti-inflammatory and cardioprotective properties by directly eliminating free radicals, as well as indirectly via antioxidant activity. In addition, melatonin may be involved in blood pressure regulation and have significant anti-atherogenic effects (8-13).
In this systematic review, cardiovascular diseases such as hypertension, myocardial infarction, ischemia, and reperfusion were selected to verify the action of melatonin, as we believe that these cardiopathies currently represent a large number of cardiovascular diseases (13,14). Our study aimed to verify the effects of melatonin in an experimental model of myocardial infarction.
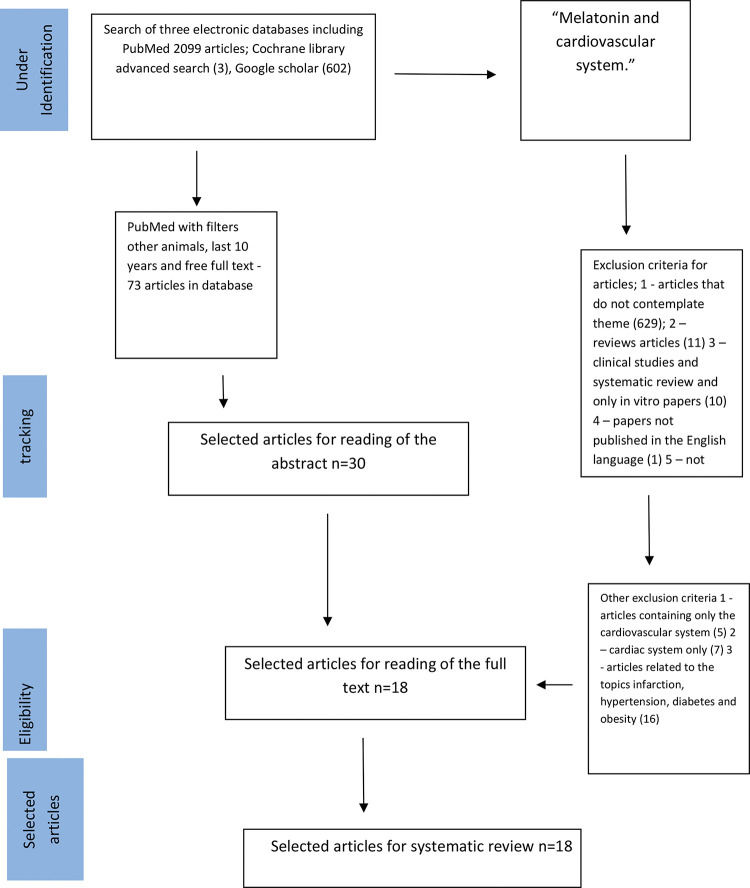
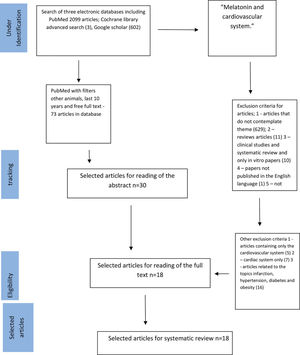
SEARCH STRATEGIESIn the present study, the search strategy was performed as described by Tawfik et al. (15). We used MEDLINE, Google Scholar, and Cochrane databases and reviewed literature published from November 2008 to June 2019; we restricted this systematic review to the last ten years, covering the latest and most relevant articles worldwide. First, we selected keywords from related articles, using Medical Subject Headings (MeSH) to identify more related keywords with similar meanings as follows: (“melatonin”) [MeSH Terms] AND (“cardiovascular system”) [MeSH Terms] [All Fields]. We then searched the three databases. Accordingly, we identified 2096 articles in PubMed using the “other animals” filter, 602 articles using Google Scholar filtering for keywords only in the title, and three articles using a Cochrane Library advanced search; the terms used were “melatonin and cardiovascular system” In addition, we reviewed retrieved articles to identify additional studies (Figure 1). This review was conducted according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (16,17).
We excluded studies with cell culture experiments, as well as pre- and post-conditioning studies. The inclusion criteria were animal studies, cell culture studies, and in vivo experiments. The control group was the melatonin group in this study. The melatonin group varied in each article, as studies persistently experimented with a melatonin group related to a drug or an event.
The process of paper retrieval and titles and abstract evaluation was conducted by two independent blinded researchers capable of compiling systematic reviews (ECV and RS), following the inclusion and exclusion criteria according to the tenets of PICO (16-19). The PICO was defined as patients in case the systematic review was performed in animals, interventions considering the administration of melatonin in animals using an experimental model of myocardial infarction, comparison, to compare the melatonin group with the control group receiving no melatonin, and outcome, which were results of administering melatonin. The selected articles were critically evaluated to determine their potential inclusion in the review. In the event of a disagreement between investigators regarding studies selected, a third reviewer was consulted (LCA).
In the present systematic review, data obtained from selected studies were tabulated, and the following characteristics were listed when present in the articles: authors' names, year of publication, animal type, sex (M/F), animal species, age (months), weight, induction model, and site injury (Table 1). Table 2 presents the following information: authors, sample size, number of groups, number of animals per group, melatonin administration, melatonin doses, and dependent variables. Table 3 lists the most frequent recommendations in preclinical research guidelines for in vivo animal experiments (18). Table 4 evaluates the study characteristics of selected controlled animal studies, with prior exercise and myocardial infarction as variables that showed a significant difference between the melatonin control group and the study group. These were classified as S for “significant difference,” and variables that did not present a significant difference were classified as NS (not significant).
Study characteristics of selected control experimental studies assessing melatonin and the cardiovascular system.
| Authors | Animal type | Animal race | Age (months) | Weight | Induction model | Site injury |
|---|---|---|---|---|---|---|
| Zhang et al. (21) | Mice | C57/B6 | − | − | Sepsis-induced cardiac dysfunctional | Cardiovascular system |
| Benova et al. (22) | Rat | Wistar | 9 months | − | Obesity | Cardiovascular system |
| Chen et al. (23) | Rat | Sprague-Dawley | − | 200-250 g | Myocardial ischemia reperfusion | Myocardial tissue |
| Liu et al. (24) | Mice | C57/B6 | 6 months | − | Myocardial infarction | Heart |
| Simko et al. (25) | Rat | Wistar | 3 months | − | hypertension | Cardiovascular system |
| Simko et al. (26) | Rat | Wistar | 3 months | hypertension | Cardiovascular system | |
| Stacchiotti et al. (27) | Mice | B6.VLEAN/OlaHsd and B6.V-Lepob/OlaHsd | 4 weeks | − | Obesity | Mitochondria of cardiomyocyte |
| Chaudagar et al. (28) | Rat | Wistar | 8 months | − | hypertension | Cardiovascular system |
| Salmanoglu et al. (29) | Rat | Wistar | − | 250-350 g | Diabetic | Liver tissue |
| Cheng et al. (30) | Rabbits | New Zealand | 4 weeks | 2.0-2.5 kg | Atherosclerosis | Aorta |
| Liu et al. (31) | Rat | Sprague-Dawley | 3 months | 280-360 g | Myocardial ischemia reperfusion | Myocardial tissue |
| Zhu et al. (32) | Rat | Sprague-Dawley | 10 weeks | 250 g | Myocardial infarction | Heart |
| Liu et al. (33) | Rat | − | − | 350-400 g | Myocardial ischemia reperfusion | Heart |
| Drobnik et al. (34) | Rat | Wistar | 4 weeks | 290-320 g | Myocardial infarction | Heart |
| Repova et al. (35) | Rat | Wistar | 3 months | hypertension | Cardiovascular system | |
| Drobnik et al. (36) | Rat | Wistar | − | 300-330 g | Myocardial infarction | Heart |
| Chen et al. (37) | Mice | Mice Gpx-/- C57BL/6 | − | − | Myocardial ischemia reperfusion in vitro | Heart |
| Petrosillo et al. (38) | Rat | Wistar | − | 250-330 g | Myocardial ischemia reperfusion | Heart |
Characteristics (samples size, number of groups, number of animals/groups, dependent variables) of selected experimental studies assessing the effects of melatonin and the cardiovascular system.
| Authors | Sample size | Number of groups | Number of animals/groups | Melatonin administration | Melatonin doses | Dependent variables |
|---|---|---|---|---|---|---|
| Zhang et al. (21) | 24 | 4 | 6 | Intraperitoneal injection | 30 mg/kg | Echo, histological analysis, creatinine kinase measurement, TUNEL analysis, western blotting. |
| Benova et al. (22) | 48 | 4 | 12 | Drinking water | 10 mg of melatonin was dissolved in 100 mL of water for 8 weeks | Heart function in Langendorff perfusion, western blot, real-time PCR, |
| Chen et al. (23) | 30 | 5 | Intraperitoneal at the reperfusion | 20 mg/kg | Echo, IS2, lactate dehydrogenase release, CMEC measurement in vitro IRI assay, western blotting, qRT-PCR, and detection of autophagosomes. | |
| Liu et al. (24) | 18 | 3 | 6 | Gavage | 50 mg/kg | Echo, histological analysis, PCR, western blot, CTRP3 detection. |
| Simko et al. (25) | 40 | 4 | 10 | Water consumption was 12-13 mL/100 g of body weight | 10 mg of melatonin was dissolved in 100 mL of water for 4 weeks | Hemodynamics measures, biometric analysis, determination of hydroxyproline, angiotensin, and aldosterone analysis. |
| Simko et al. (26) | 66 | 6 | 11 | Drinking water adjustment to daily water consumption to ensure the correct dosage | 10 mg/kg/day for 6 weeks | Hemodynamics measures, determination of hydroxyproline, NO synthase activity, oxidative load measurement, and western blotting of NF-ΚB. |
| Stacchiotti et al. (27) | 40 | 4 | 10 | 5th to 13th weeks of life/drinking water | 100 mg/kg/day for 8 weeks | Histomorphometric evaluations, nuclear cardiomyocyte morphometry, mitochondrial and immunohistochemical analysis. |
| Chaudagar et al. (28) | 24 | 4 | 6 | Drinking water | 10 mg/kg/day for 67 days | Hemodynamics measures, biometric analysis, and NO assays. |
| Salmanoglu et al. (29) | 35 | 6 | − | Oral gavage | 10 mg/kg/day for 2 weeks | Vasocontractile response, measurement of total cholesterol, LDL, HDL, glucose, NO, and insulin, MDA assay, and tissue antioxidant levels. |
| Cheng et al. (30) | 60 | 3 | 20 | − | 20 mg/kg for 4 weeks | Immunohistochemical analysis, HE staining, western blot analysis, and qRT-PCR. |
| Liu et al. (31) | 60 | 5 | 12 | Intravenous injection immediately after reperfusion | 10 mg/kg | IF2, myocardial ultrastructure, western blotting and determination of the opening degree of MPTPs. |
| Zhu et al. (32) | − | − | − | Melatonin stem cells were treated for 24 hours | 5 μM | Measurements of cell culture antioxidant properties, apoptosis, analysis of paracrine factors, LV functions, histology. |
| Liu et al. (33) | 60 | 6 | 12 | Intraperitoneal injection | Group I: 2.5 mg/kg, Group II: 5 mg/kg, Group III: 10 mg/kg | Hemodynamics measures, apoptosis, electron microscope examination, analysis on mitochondria. |
| Drobnik et al. (34) | 21 | 3 | 7 | Drinking water for 6 weeks | 10 mg/kg | Collagen determination, estimation of glycosaminoglycans, electron microscope examination. |
| Repova et al. (35) | 40 | 4 | 10 | Drinking water for 6 weeks | 10 mg/kg | Collagen determination, hemodynamics measures. |
| Drobnik et al. (36) | 60 | 5 | 12 | Intraperitoneal injection for 4 weeks | Group 1: 300 μg/100 g b.w. Group 4: 3 mg/100 g.b.w. Group 5: 1.5 mg/100 g.b.w. | Estimation of lipid peroxidation, collagen determination, estimation of glycosaminoglycans. |
| Chen et al. (37) | − | − | − | Intraperitoneal injection 30 min before harvesting the hear for in vitro preparation | 150 μg/kg | Cardiac function, hemodynamics measures, lactate dehydrogenase released, apoptosis, immunohistochemistry. |
| Petrosillo et al. (38) | 42 | 6 | 7 | Krebs-Henseleit solution for isolated heart | 50 μM | Infarct size, lactate dehydrogenase released, hemodynamics measures, analysis on mitochondria. |
IS1, measurement of infarct size by echocardiography; IS2, measurement of infarct size by Evans Blue or tetrazolium; echo, echocardiography measurements; CMEC, cardiac microvascular endothelial cells; IRI, ice recrystallization inhibition; CTRP3, C1q TNF Related Protein 3; NO, nitric oxide; LDL, low-density lipoprotein; HDL, high-density lipoprotein; MDA, malondialdehyde; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; LV, left ventricular; MPTP, mitochondrial permeability transition pore; NF-ΚB, Nuclear factor-kappa B; g.b.w., gross body weight; HE, hematoxylin-eosin.
Most frequent recommendations appearing in preclinical research guidelines for in vivo animal experiments [Hendersen et al. (18)].
| Validity type | Recommendation Category | Examples |
|---|---|---|
| Internal | Choice of sample size | Power calculation, larger samples sizes |
| Randomized allocation of animals to treatment | Various methods of randomization | |
| Blinding of outcome assessment | Blinded measurement or analysis | |
| Flow of animals through an experiment | Recording animals excluded from treatment through to analysis | |
| Selection of appropriate control groups | Using negative, positive, concurrent, or vehicle control groups | |
| Study of dose-response relationships | Testing above and below optimal therapeutic dose | |
| Construct | Characterization of animal properties at baseline | Characterizing inclusion/exclusion criteria, disease severity, age or sex |
| Matching model to the human manifestation of the disease | Matching mechanism, chronicity or symptoms | |
| Treatment response along a mechanistic pathway | Characterizing pathway in terms of molecular biology, histology, physiology or behavior | |
| Matching outcome measures to clinical settings | Using functional or non-surrogate outcome measures | |
| Matching model to the age of patients in clinical settings | Using aged or juvenile animals | |
| External | Replication in different models of the same disease | Different transgenic strains or lesion techniques |
| Independent replication | Different investigators or research groups | |
| Replication in different species | Rodents and nonhuman primates | |
| Research program | Inter-study standardization of experimental design | Coordination between independent research groups |
Most frequent recommendations in preclinical research guidelines for in vivo animal experiments [Henderson et al. (18)].
| Validity type | Recommendation Category | Studies | n (Percent of guidelines Citing) |
|---|---|---|---|
| Internal | Choice of sample size | Zhang et al. (21); Benova et al. (22); Simko et al. (25); Simko et al. (26); Stacchiotti et al. (27); Cheng et al. (30); Liu et al. (31); Liu et al. (33); Repova et al. (35); Drobnik et al. (36); Petrosillo et al. (38). | 61.11% |
| Randomized allocation of animals to treatment | Zhang et al. (21); Chen et al. (23); Simko et al. (25); Simko et al. (26); Salmanoglu et al. (29); Cheng et al. (30); Liu et al. (31); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36). | 61.11% | |
| Blinding of outcome assessment | Zhang et al. (21); Benova et al. (22); Chen et al. (23); Simko et al. (25); Liu et al. (24); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Salmanoglu et al (29); Cheng et al. (30); Liu et al. (31); Zhu et al. (32); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Chen et al. (37); Petrosillo et al. (38). | 100% | |
| Flow of animals through an experiment | − | − | |
| Selection of appropriate control groups | Zhang et al. (21); Chen et al. (23); Simko et al. (25); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Salmanoglu et al. (29); Cheng et al. (30); Liu et al. (31); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Petrosillo et al. (38). | 77.77% | |
| Study of dose-response relationships | Chen et al. (23); Simko et al. (25); Simko et al. (26); Chaudagar et al. (28); Cheng et al. (30); Liu et al. (31); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Petrosillo et al. (38). | 61.11% | |
| Construct | Characterization of animal properties at baseline | Zhang et al (21); Benova et al. (22); Chen et al. (23); Simko et al. (25); Liu et al (24); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Salmanoglu et al. (29); Cheng et al. (30); Liu et al. (31); Zhu et al. (32); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Chen et al. (37); Petrosillo et al. (38). | 100% |
| Matching model to the human manifestation of the disease | Zhang et al. (21); Benova et al. (22); Chen et al. (23); Simko et al. (25); Simko et al. (26); Salmanoglu et al. (29); Cheng et al. (30); Liu et al. (31); Zhu et al. (32); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Chen et al. (37); Petrosillo et al. (38). | 88.88% | |
| Treatment response along a mechanistic pathway | Zhang et al. (21); Benova et al. (22); Chen et al. (23); Simko et al. (25); Liu et al. (24); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Salmanoglu et al. (29); Cheng et al. (30); Liu et al (31); Zhu et al. (32); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Chen et al. (37); Petrosillo et al. (38). | 100% | |
| Matching outcome measures to clinical settings | Chen et al. (23); Simko et al. (25); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Salmanoglu et al. (29); Cheng et al. (30); Liu et al. (31); Liu et al. (33); Repova et al. (35); Chen et al. (37); Petrosillo et al. (38). | 66.66% | |
| Matching model to the age of patients in clinical settings | Zhang et al. (21); Benova et al. (22); Chen et al. (23); Simko et al. (25); Liu et al. (24); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Salmanoglu et al. (29); Liu et al. (31); Zhu et al. (32); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Chen et al. (37); Petrosillo et al. (38). | 100% | |
| External | Replication in different models of the same disease | − | − |
| Independent replication | Zhang et al. (21); Chen et al. (23); Simko et al. (25); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Salmanoglu et al. (29); Cheng et al. (30); Zhu et al. (32); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Chen et al. (37); Petrosillo et al. (38). | 77.77% | |
| Replication in different species | Zhang et al. (21); Benova et al. (22); Chen et al. (23); Simko et al. (25); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Salmanoglu et al. (29); Liu et al. (31); Zhu et al. (32); Liu et al. (33); Drobnik et al. (34); Repova et al. (35); Drobnik et al. (36); Chen et al. (37); Petrosillo et al. (38). | 88.88% | |
| Research program | Inter-study standardization of experimental design | Simko et al. (25); Simko et al. (26); Stacchiotti et al. (27); Chaudagar et al. (28); Zhu et al. (32); Repova et al. (35); Chen et al. (37). | 38.88% |
RevMan (version 5.3; Cochrane Collaboration, Oxford, UK) was used to perform the meta-analysis. The random-effects model was used to account for the heterogeneity.
Statistical analysisMean values and standard deviation between studies, presented as the mean difference (MD) of post-intervention values after calculating the inverse variance, were employed to verify the magnitude of the protection afforded by melatonin (19). In addition, heterogeneity was assessed using Cochran's Q and I2 tests, followed by visual inspection of the graph. The analyses were performed using the RevMan software (version 3.3.1) (20).
RESULTSFigure 1 presents the search process, identification, and selection of articles. Based on our search strategies, 73 articles were retrieved from 2099 identified articles in PubMed using the “other animals” filter; among these, 18 were selected after reading the title and abstract. In addition, we selected three articles from BIREME and 0 articles from the Cochrane database. The inclusion and exclusion criteria are described in Figure 1 (21-38). Articles were primarily excluded when assessments were performed in human subjects, apart from being unrelated to components of PICO; we mainly focused on experimental animal studies.
In Table 4, we employed the criteria of Henderson et al. (18). We found that 61.11% (21,22,25-27,30,31,33,35,36,38) of selected studies had an appropriate sample size and 61.11% (21,23,25,26,27,30,31,33-36) had randomized animals, according to their materials and methods. All articles were blinded to the outcome assessment (21-38). We could not determine the criterion underlying the flow of animals through experiments, as no explicit statement regarding the same was available in the materials and methods. We observed that 77.77% of articles selected appropriate control groups (21,23,25-31,34,36) and 61.11% (23,25,26,28,30-32,34,36,38) had well-defined dose-response relationships. Moreover, all studies analyzed (21-38) had standard characterizations of animal properties at baseline. Overall, 89% of studies had employed an appropriate animal model that simulated the human manifestation of the disease (21-23,25-27,29-38). All studies that examined treatment responses according to a known mechanism (21-38) had characteristics within the requested standards. Only 67% (23-25,31,33,35,37,38) of selected manuscripts were within the range of the standard model of patient age in clinical settings. All studies did not follow other standard application models. In replicating different models of the same disease, 78% of studies (21-23,25-2930,32,34-38) were independently replicated, whereas 88.88% (21-23,25-29,31-38) were replicated in different species. For studies where the objective was inter-study standardization of an experimental design, 39% (25-28,32,35,37) reached this standard (Table 4).
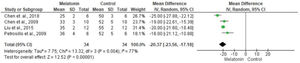
In Table 5, we analyzed the study characteristics of selected controlled animal studies. Accordingly, we obtained the following results according to each experiment performed in articles examined in this systematic review. Table 5 presents experiments in which melatonin significantly improved the investigated variable (marked as S), as well as those where melatonin showed no significant improvements (NS). Chen et al. (23), Liu et al. (31), and Petrosillo et al. (38) reported that melatonin significantly decreased infarct size. Zhang et al. (21) and Liu et al. (24) reported that melatonin improved echocardiographic measurements. Furthermore, studies by Benova et al. (22), Liu et al. (24), Simko et al. (25), Simko et al. (26), Liu et al. (33), Repova et al. (35), and Chen et al. (37) showed that melatonin had a positive effect on hemodynamic variables. In addition, we observed that the effects of melatonin were not significantly different from those reported in the study by Chaudagar et al. (28). These findings indicated the substantial benefit of using melatonin to stabilize hemodynamic parameters. Moreover, Zhu et al. (32) and Chen et al. (37) revealed that melatonin improved left ventricular cardiac function (Figure 2, Table 5).
Study characteristics of selected controlled animal studies assessing melatonin and the cardiovascular system.
| Authors | Assessments | |||||
|---|---|---|---|---|---|---|
| Zhang et al. (21) | S | S | S | S | S | S |
| Echocardiography measurements | Apoptosis analysis | Western blotting | Creatinine kinase measurement | Immunohistochemical analysis | Detection of autophagosomes | |
| Benova et al. (22) | S | S | S | S | NS | |
| Biometric analysis | Western blotting | qRT-PCR | Hemodynamics measures | |||
| Chen et al. (23) | S | S | S | S | S | S |
| Measurements of the infarct size | Measurement of lactate dehydrogenase | Measures of CMEC in vitro IRI assay | Western blotting | qRT-PCR | Detection of autophagosomes | |
| Liu et al. (24) | S | S | S | S | S | |
| Echocardiography measurements | Hemodynamics measurements | Apoptosis analysis | Western blotting | qRT-PCR | ||
| Simko et al. (25) | S | S | NS | S | NS | |
| Biometric analysis | Hemodynamics measures | Determination of hydroxyproline | Angiotensin analysis | Aldosterone analysis | ||
| Simko et al. (26) | NS | S | S | S | S | |
| Hemodynamics measures | Determination of hydroxyproline | NO synthase activity | Oxidative load | Measurement and western blotting of NF-ΚB | ||
| Stacchiotti et al. (27) | S | S | S | S | ||
| Histomorphometrically evaluations | Nuclear cardiomyocyte morphometric | Mitochondrial analysis | Immunohistochemical analysis | |||
| Chaudagar et al. (28) | NS | S | S | |||
| Hemodynamics measures | Biometric analysis | NO assays | ||||
| Salmanoglu et al. (29) | NS | NS | S | S | NS | |
| Vasocontractile response | Measures of total cholesterol, LDL, HDL | NO assays | MDA assay | Measurements of tissue antioxidant levels | ||
| Cheng et al. (30) | S | S | S | |||
| Immunohistochemical analysis | Western blotting | qRT-PCR | ||||
| Liu et al. (31) | S | S | S | |||
| Measurements of the infarcted size | Western blotting | Determination of the opening degree of MPTPs | ||||
| Zhu et al. (32) | S | S | S | |||
| Measurements of cell cultures antioxidant properties | Apoptosis analysis | LV functions | ||||
| Liu et al. (33) | S | S | S | S | ||
| Hemodynamics measures | Apoptosis analysis | Electron microscope examination | Analysis on mitochondria | |||
| Drobnik et al. (34) | S | S | S | |||
| Determination of collagens | Determination of glycosaminoglycans | Electron microscope examination | ||||
| Repova et al. (35) | S | S | ||||
| Hemodynamics measures | Determination of collagen | |||||
| Drobnik et al. (36) | S | NS | S | |||
| Estimation of lipid peroxidation | Determination of collagen | Determination of glycosaminoglycans | ||||
| Chen et al. (37) | S | S | S | S | S | |
| Cardiac function | Hemodynamics measures | Lactate dehydrogenase | Apoptosis analysis | Immunohistochemistry | ||
| Petrosillo et al. (38) | S | S | S | S | ||
| Measurements of the infarct size | Lactate dehydrogenase | Hemodynamics measures | Analysis on mitochondria | |||
S, statistically significant; NS, not significant; CMEC, cardiac microvascular endothelial cells; IRI, ice recrystallization inhibition; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; NO, nitric oxide; NF-ΚB, Nuclear factor-kappa B; LDL, low-density lipoprotein; HDL, high-density lipoprotein; MDA, malondialdehyde; LV, left ventricular.
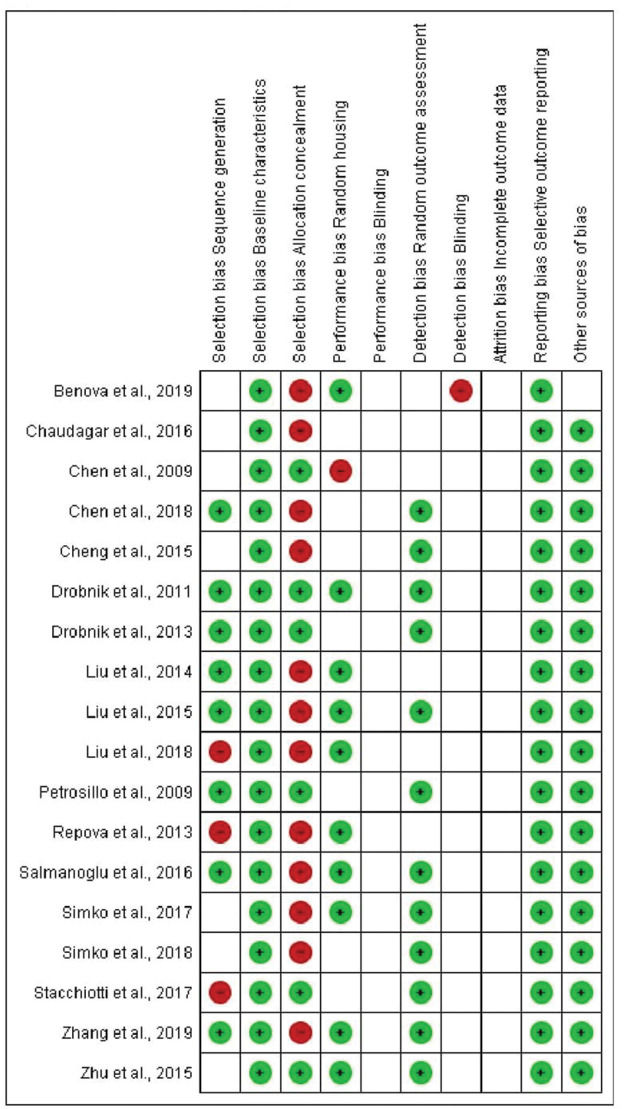
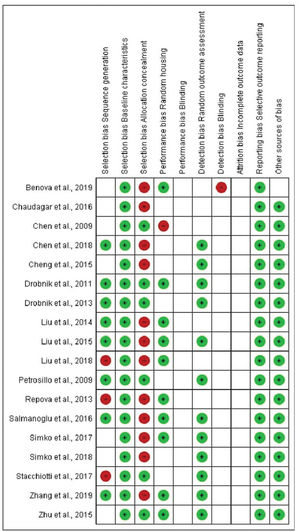
Representation of the SYRCLE's risk of bias tool for animal studies. Hooijmans et al. (43).
Zhang et al. (21), Liu et al. (24), Simko et al. (26), Salmanoglu et al. (29), Zhu et al. (32), and Chen et al. (37) revealed that melatonin had positive effects on the rate of apoptosis (Table 5). Melatonin showed positive effects in studies examining western blotting of various proteins and quantitative reverse transcription-polymerase chain reaction (qRT-PCR), including those by Zhang et al. (21), Benova et al. (22), Chen et al. (37), Liu et al. (31), and Liu et al. (24) (Table 5). Drobnik et al. (34) and Repova et al. reported that melatonin reportedly reduced collagen deposition (35) (Table 5). Immunohistochemical analyses were performed by Zhang et al. (21), Stacchiiotti et al. (27), Cheng et al. (30), Liu et al. (33), Drobnik et al. (34), and Cheng et al. (37), revealing that melatonin consistently yielded a positive result (Table 5). Melatonin showed benefits in biometric analyses, as determined by Benova et al. (22), Simko et al. (25), and Chaudagar et al. (28) (Table 5).
Furthermore, melatonin showed beneficial effects on autophagosome evaluation, lactose dehydrogenase measurements, angiotensin, and aldosterone, nitric oxide levels, and mitochondrial analysis, as determined by Zhang et al. (21), Chen et al. (23), Simko et al. (25), Liu et al. (31), Liu et al. (33), Chen et al. (37), and Petrosillo et al. (38) (Table 5).
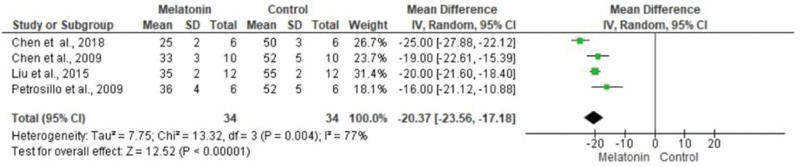
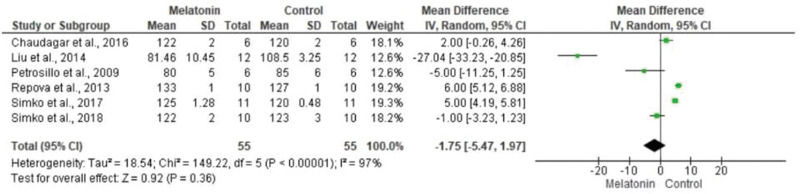
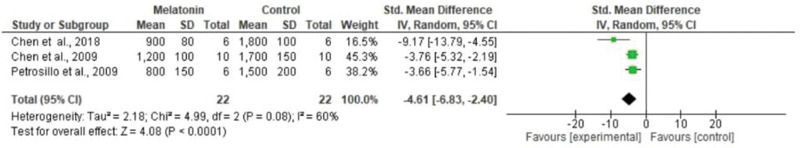
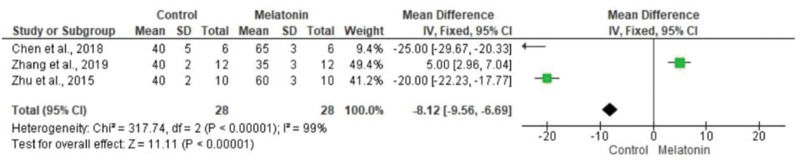
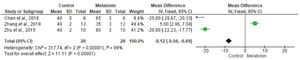
The meta-analysis revealed a statistically significant decrease in infarct size (MD -20.37 [-23.56, -17.18]). However, there was no statistical difference in systolic pressure between articles analyzed (MD -1.75 [-5.47, 1.97]). In articles analyzing lactate dehydrogenase, a statistically significant decrease in the levels of this enzyme was noted in animals in melatonin groups (MD -4.61 [-6.83, -2.40]). With regard to the ejection fraction, two articles showed improvement in melatonin-treated groups. Another study analyzed the influence of melatonin in infarcted animals with the same ejection fraction; however, this parameter was not statistically significant in the meta-analysis (MD -8.12 [-9.56, -6.69]) (Figure 3).
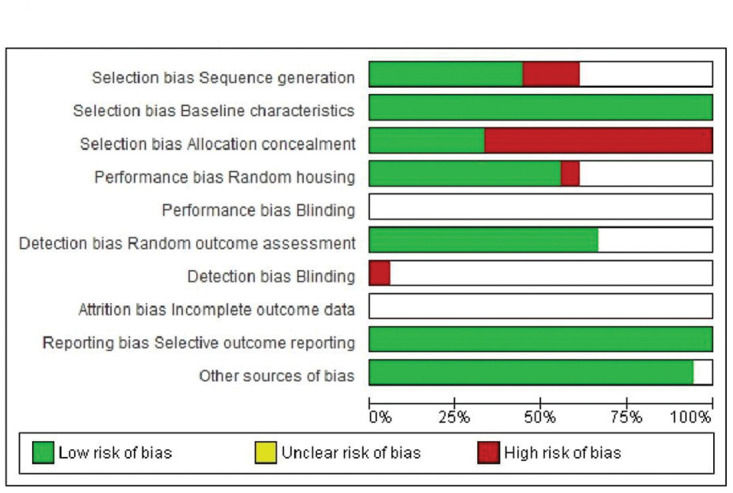
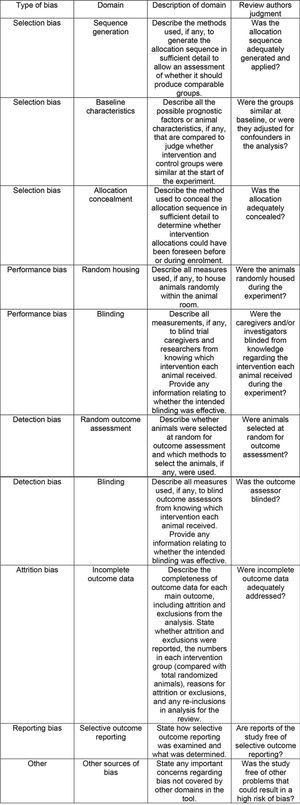
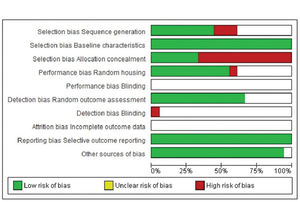
In terms of selection bias, the results were well-balanced between low risk, no clear risk, and high risk of bias. All studies presented a low risk of bias in the baseline variable characteristics. On analyzing allocation concealment, most selected articles had a high risk, and a little less than half presented a low risk of bias. The randomization parameter was also fairly balanced between low risk, no clear risk, and high risk of bias. On analyzing random outcome assessment, most studies (more than 50%) had a low risk of bias, and some presented an unclear risk of bias. On analyzing blinding bias, most articles were unclear as to whether investigators were blinded. The articles presented a low risk of bias in the results of incomplete outcome data (Figure 4 and 5).
This systematic review revealed that melatonin has various beneficial effects on the cardiovascular system; these effects include decreased infarct size, improved cardiac function and cellular oxidation functions, reduced apoptosis, and healthier cellular histomorphology.
In the present review, studies that analyzed echocardiographic measures exhibited melatonin benefits such as decreased infarct size, improved ejection fractions, improved systolic and diastolic diameters, and ameliorated recovery rates of cardiac function (24), while also favoring the treatment of cardiac hypertrophy and hearts that experienced myocardial infarction, ischemia, or reperfusion (21-38). On analyzing hemodynamic and biometric variables, melatonin appeared to confer significant benefits, such as improvements in systolic pressure, positive pressure derivative, lower left ventricular end-diastolic pressure, reduced left ventricular weight in relation to the total heart weight, and improved lung water content (22-25,28,35,38). Experimental models of obesity, hypertension, and other cardiovascular diseases reinforce the scientific practice of adopting animal models and assessing results prior to human application. These preclinical results indicate the effect of melatonin on the examined cardiovascular diseases.
Reportedly, melatonin is an important anti-apoptotic agent in various tissues, reducing calcium uptake, mitigating reactive oxygen species generation, and decreasing the levels of pro-apoptotic proteins, such as Bax (39). In addition, melatonin destabilizes hypoxia-induced hypoxia-inducible factor (HIF)-1α protein expression. Moreover, melatonin suppresses HIF-1α transcriptional activity under hypoxic conditions, resulting in vascular endothelial growth factor expression (40). Melatonin also confers anti-inflammatory effects on the cardiovascular system (41). Furthermore, a systematic review and recent meta-analysis have identified that melatonin supplementation facilitates blood pressure regulation (42).
Melatonin has substantial benefits in the heart, involving various proteins (including superoxide dismutase [SOD], catalase [CAT], and glutathione peroxidase [Gpx]), while also improving the apoptosis rate. These findings were determined using several techniques, including western blotting analysis of BCL and Bx expression and the TUNEL assay, which measured the decrease in the level of apoptosis in myocardial cells when melatonin was added (23-25,29,32). Other important variables analyzed following melatonin administration in the cardiovascular system were lactate dehydrogenase levels, mitochondrial analysis, lipid peroxidation, glycosaminoglycan, collagen level reduction, culture measurements, the antioxidant action of cells, opening gradient of mitochondrial channels, improvement in vasoconstriction, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and cholesterol level measurements, nitric oxide synthase level measurements, histomorphometric evaluations, determination of hydroxyproline levels, and assessment of autophagosomes (21-38).
The main novelty of this study is that it highlights the benefits assimilated by melatonin in experimental models of myocardial infarction, such as improved ejection fraction. Apart from limitations such as differences between animal organisms and humans, experimental research in Brazil is often restricted due to limited funding for animal studies when compared with human trials. In addition, results from animal studies fail to precisely correlate with the experience of testing melatonin or other substances in an environment that differs from the human body. Another limitation that must be considered is the nature of systematic reviews, which examine non-published data and data previously published by other authors, thus hindering novel scientific findings.
CONCLUSIONNotably, this systematic review is based on animal experiments. Melatonin may impact the cardiovascular system, including experimental myocardial infarction, and further studies are necessary to determine its use in clinical settings for treating cardiovascular diseases.
AUTHOR CONTRIBUTIONSVeiga ECA contributed substantially to the study conception and design, definition of intellectual content, was involved in literature search, data analysis, statistical analysis, and manuscript preparation, drafting and critical review for important intellectual content, and approved the final manuscript version to be published. Simões RS, Caviola LL, Abreu LC and Cavalli RC were involved in data analysis and statistical analysis, manuscript drafting and critical review for important intellectual content, and approved the final manuscript version to be published. Cipolla-Neto J, Baracat EC and Soares Junior JM substantially contributed to the study conception and design, definition of intellectual content, were involved in manuscript preparation, drafting and critical review for important intellectual content, and approved the final manuscript version to be published.
The author Cipolla-Neto is financed by FAPESP (2014/50457-0), and the authors Veiga and Cavalli, as well as all other authors, are financed by CNPq and CAPES (Brasíla-Br; financing number CNPq 301293 / 2018-0).
No potential conflict of interest was reported.