Brain death is typically followed by autonomic changes that lead to hemodynamic instability, which is likely associated with microcirculatory dysfunction and inflammation. We evaluated the role of the microcirculation in the hemodynamic and inflammatory events that occur after brain death and the effects of autonomic storm inhibition via thoracic epidural blockade on mesenteric microcirculatory changes and inflammatory responses.
METHODS:Male Wistar rats were anesthetized and mechanically ventilated. Brain death was induced via intracranial balloon inflation. Bupivacaine (brain death-thoracic epidural blockade group) or saline (brain death group) infusion via an epidural catheter was initiated immediately before brain death induction. Sham-operated animals were used as controls (SH group). The mesenteric microcirculation was analyzed via intravital microscopy, and the expression of adhesion molecules was evaluated via immunohistochemistry 180 min after brain death induction.
RESULTS:A significant difference in mean arterial pressure behavior was observed between the brain death-thoracic epidural blockade group and the other groups, indicating that the former group experienced autonomic storm inhibition. However, the proportion of perfused small vessels in the brain death-thoracic epidural blockade group was similar to or lower than that in the brain death and SH groups, respectively. The expression of intercellular adhesion molecule 1 was similar between the brain death-thoracic epidural blockade and brain death groups but was significantly lower in the SH group than in the other two groups. The number of migrating leukocytes in the perivascular tissue followed the same trend for all groups.
CONCLUSIONS:Although thoracic epidural blockade effectively inhibited the autonomic storm, it did not affect mesenteric hypoperfusion or inflammation induced by brain death.
Organ transplantation is a unique treatment alternative for many critically ill patients, and one major source of organs is brain-dead patients. Brain death (BD) induces a series of hemodynamic, hormonal, and inflammatory alterations that can deteriorate the viability of organs 1–3.
Parasympathetic activation associated with progressive bradycardia and hypotension is generally observed after the initial trauma that leads to BD. If the injury involves the most distal midbrain, the vagal cardiac motor center is destroyed, and parasympathetic activity is stopped. As a consequence, sympathetic activity is not regulated by the parasympathetic system, resulting in a classical autonomic storm characterized by tachycardia and hypertension. Finally, a profound reduction in sympathetic outflow leads to hemodynamic instability exacerbated by hypovolemia, dysregulation of the peripheral vasculature, and vasodilatation 4.
In animal models of BD, a balloon catheter placed in the intracranial cavity is inflated to increase the local pressure, leading to an immediate increase in the norepinephrine and epinephrine concentrations, which are associated with tachycardia and hypertension followed by vasoplegia and hypotension 5–7. The prompt appearance of a hypertensive peak followed by an immediate decrease in arterial blood pressure below the baseline level has been observed in models of rapidly increasing intracranial pressure 8,9. Alternatively, models of slowly increasing intracranial pressure show a gradual elevation of mean arterial pressure, which is also followed by a hypotensive episode 10.
Controversial effects documented using different methods of autonomic storm inhibition hamper the understanding of the influence of sympathetic activity on transplantable organs. Administering intravenous propranolol, xylazine, and labetalol 11–13 inhibits the sudden increase in mean arterial pressure after BD induction, resulting in decreased damage to the myocardial tissue and reduced production of multiple myocardial gene products. In contrast, in a surgical model of sympathectomy in baboons, Novitzky et al. 14 found that this procedure did not abolish the hypertensive crisis, indicating that the performance of this surgical procedure is not sufficient to block the autonomic storm. In addition, Silva et al. 9 demonstrated that thoracic epidural anesthesia effectively blocks the autonomic storm but does not alter the expression of inflammatory mediators in the myocardial tissue.
The hemodynamic instability observed in BD is generally associated with ischemia and tissue hypoperfusion, which compromise the viability of the organs that are suitable for transplantation 2. Previous studies have shown that BD leads to substantial impairment of microcirculation in the liver and the pancreas 15,16. Furthermore, our group observed similar alterations triggered by BD in the rat mesenteric microcirculation 8. In particular, mesenteric hypoperfusion immediately occurred after BD induction, and this phenomenon persisted for up to three hours thereafter. This hypoperfusion was associated with elevated expression of endothelial cell adhesion molecules and increased leukocyte migration to the perivascular tissues such as in mesentery, lung, and liver tissue 8,17.
Despite the clear importance of the microcirculation for optimizing the therapy for circulatory shock of various etiologies, the influence of autonomic storm blockade on microcirculatory changes after BD has not been investigated. Therefore, the present study was conducted to assess the role of the microcirculation in hemodynamic and inflammatory events that occur after BD induction and to investigate the effects of sympathetic system inhibition via thoracic epidural anesthesia. Microcirculatory changes, including perfusion and leukocyte-endothelial interactions, were analyzed via intravital microscopy of the mesentery. Local and systemic inflammatory markers were also evaluated.
MATERIAL AND METHODSMale Wistar rats (300±50 g) were used according to experimental protocols approved by the Animal Subject Committee of the Instituto do Coração (InCor) do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo. The animals were randomly allocated to three groups: sham-operated (SH group, n=12), brain-dead non-treated (BD group, n=12), and brain-dead under thoracic epidural blockade (TEB) (BD-TEB group, n=12). The animals were evaluated 180 min after the conclusion of the surgical procedures.
Brain death model and thoracic epidural anesthesiaAll animals were anesthetized in a chamber using isoflurane (5%), intubated, and mechanically ventilated (Harvard Apparatus, Holliston, MA, USA) with 100% FiO2 (tidal volume: 10 mL/kg, 70 breaths/min). Sedation was maintained via continuous inhalation of 2% isoflurane. The carotid artery was cannulated for blood pressure monitoring and blood sampling. The jugular vein was cannulated for continuous infusion of saline solution (2 mL/h) to minimize dehydration. No intravenous drugs were administered.
BD induction was performed as previously described 9,17. Briefly, a Fogarty 4-French catheter (Baxter Health Care, Deerfield, IL, USA) was placed in the intracranial cavity through a drilled parietal burr hole. The balloon catheter was rapidly inflated with 0.5 mL of saline solution to increase the intracranial pressure until BD was confirmed based on maximal pupil dilatation and the absence of the corneal and respiratory reflexes. Anesthesia with isoflurane was discontinued after BD induction.
A 10-French polyethylene catheter was placed in the epidural space through an incision over the T11–L1 vertebrae and was advanced to the T5 level. The BD-TEB group received a bolus infusion of 20 µL of 0.5% bupivacaine 5 min before BD induction, followed by continuous infusion (15 µL/h) for 3 h. The animals in the BD group were administered equivalent volumes of saline solution. No drugs were administered to the rats in the SH group.
Blood gas, electrolyte, and lactate measurements and white blood cell countsThe blood gas, electrolyte, and lactate levels were measured in blood samples obtained from the carotid artery at baseline (0 min) and 180 min after the surgical procedures using a gas analyzer (Radiometer ABL 555, Radiometer Medical, Copenhagen, Denmark). White blood cell (WBC) counts were determined in blood samples obtained from the cut tip of the tail vein at the same time points. The total WBC counts were determined using a Neubauer chamber. Differential cell counting was performed on stained films via oil immersion microscopy. A total of 100 cells were counted and classified according to standard morphological criteria.
Intravital microscopy of the mesenteric microcirculationIntravital microscopy of the mesenteric microcirculation was performed as previously described 8. Briefly, an abdominal midline incision was performed 180 min after BD induction, and the distal ileum and its accompanying mesentery were exposed for in vivo microscopic examination of the microcirculation. The animals were maintained on a specially designed stage warmed with circulating 37°C water. The mesentery was continuously perfused with warm (37°C) Krebs-Henseleit solution saturated with a mixture of gases (95% N2 and 5% CO2). A color camera (AxioCam HSc, Carl Zeiss, München-Hallbergmos, Germany) was connected to a triocular microscope (Axioplan 2, Carl Zeiss), and the microcirculatory variables were analyzed using Axiovision 4.1 imaging software (Carl Zeiss). The vascular density in a 1-mm2 area on the computer screen was assessed using a 10x light microscope objective. The flow, defined as continuous or intermittent/absent, was analyzed in vessels with a diameter <30 µm. Five fields of view were selected for each animal. To evaluate leukocyte-endothelial interactions, three post-capillary venules with diameters ranging from 15 to 25 µm were selected for each animal. The number of rolling leukocytes was calculated as the mean number of WBCs passing a designated line perpendicular to the venular axis per 5 min. The adherent cells (i.e., leukocytes that remained stationary on the venular endothelium for more than 30 s) were counted in a 100 µm segment of the vessel. The number of leukocytes that accumulated in the connective tissue adjacent to the selected post-capillary venule was determined in a standard area of 5,000 µm2. Three fields of view for each microvessel were examined.
Evaluation of intercellular adhesion molecule 1 expression via immunohistochemistryThe animals were exsanguinated from the abdominal aorta, and the mesentery was removed, immersed in hexane, and frozen in liquid nitrogen. Serial slices of the mesentery (8 µm) were placed on glass slides coated with organosilane (Sigma Chemical Co., St. Louis, MO, USA). The samples were fixed in acetone, and SuperBlock buffer (Pierce Biotechnology, Rockford, IL, USA) was used to block nonspecific sites. For the immunodetection of intercellular adhesion molecule 1 (ICAM-1) on mesenteric microvessels, tissue sections were incubated overnight at 4°C in a biotin-conjugated anti-rat ICAM-1 (CD54) monoclonal antibody (Seikagaku, Tokyo, Japan) diluted 1:50 in phosphate-buffered saline (PBS) containing 0.3% Tween-20. After washing the slides with PBS, the sections were incubated for 1 h at room temperature in streptavidin-fluorescein (Amersham Pharmacia Biotech, London, UK) diluted 1:200 in PBS. After washing with PBS, the samples were treated with Vectashield mounting medium containing propidium iodide (Vector Laboratories, Burlingame, CA, USA) to preserve their fluorescence. The system used for image acquisition included a DS-Ri1 digital camera (Nikon, Tokyo, Japan) connected to a fluorescence microscope (Nikon) controlled with NIS-Elements-BR software (Nikon). The results are presented as the mean fluorescence intensity.
Serum concentrations of cytokines and corticosteroneBlood samples obtained from the abdominal aorta were centrifuged (1,500 g, 25°C), and the serum concentrations of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-10, and corticosterone were determined using enzyme-linked immunosorbent assay (ELISA) kits as recommended by the manufacturers (cytokines: R&D Systems, Minneapolis, MN, USA; corticosterone: Cayman Chemical, Ann Arbor, MI, USA).
Statistical analysisAll data are presented as the means ± standard error of the mean (SEM). The overall group differences were compared via two-way analysis of variance (ANOVA) using group and time as the factors followed by a post hoc Bonferroni test or via a one-way ANOVA followed by a post hoc Bonferroni test. Adjusted p values for multiple comparisons were calculated, and a p-value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using Graph-Pad Prism 6.1.
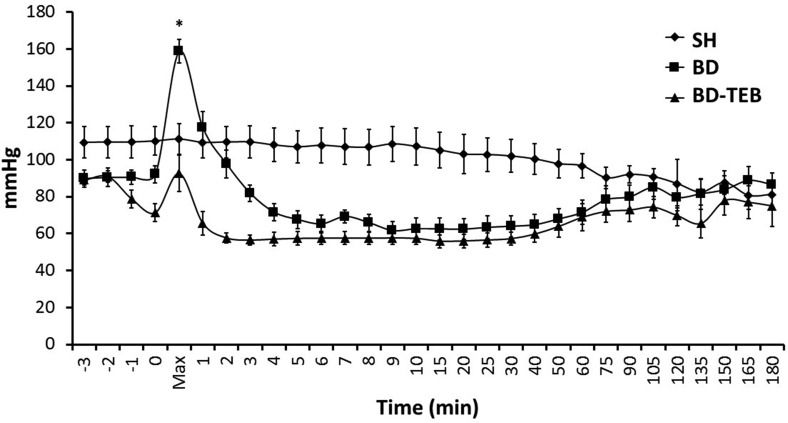
RESULTSHemodynamic parametersThe rats in the BD group exhibited a sudden increase in mean arterial pressure over the first minute following catheter inflation. In contrast, the rats treated with TEB did not exhibit this hypertensive episode after BD induction. The mean arterial pressure then decreased below the baseline level in both groups. After 75 min, the mean arterial pressure was restored to normal levels, and no difference in mean arterial pressure was observed between the groups. In the SH rats, the mean arterial pressure did not change over time (Figure 1). There were no significant differences in the arterial blood gas, electrolyte, or lactate levels between the groups (data not shown).
Mean arterial pressure of sham-operated rats (SH), brain-dead non-treated rats (BD), and brain-dead rats under thoracic epidural blockade (BD-TEB) 180 minutes after the surgical procedures. The animals were monitored over time. The data are presented as the means±SEM; ANOVA p<0.001; *p<0.05 vs. the other groups.
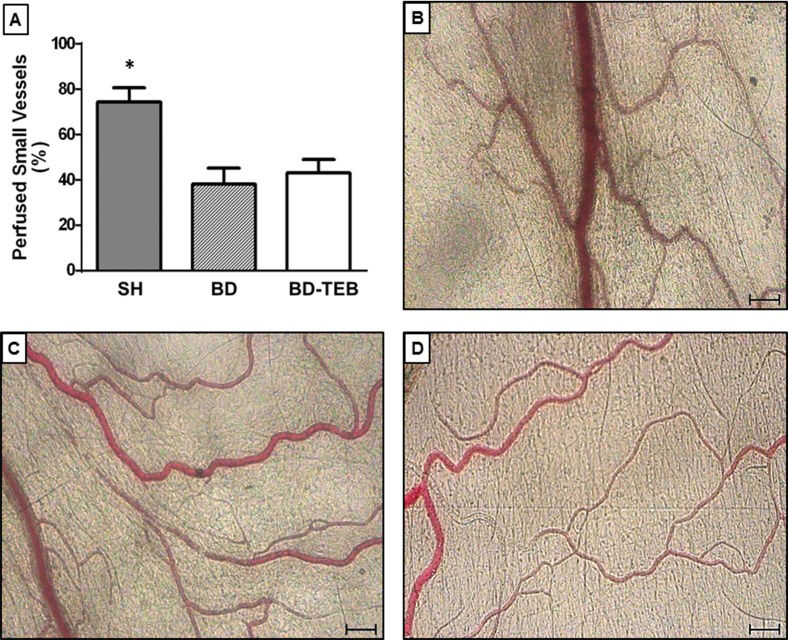
The average proportion of perfused small vessels in the BD group was 39% compared to 74% in the SH rats. The treatment of BD rats with TEB did not alter mesenteric hypoperfusion (43%), as illustrated in Figure 2 and the supplementary videos.
Intravital microscopy of the rat mesenteric microcirculation. A, The percentages of perfused small vessels (<30 μm) in sham-operated rats (SH), brain-dead non-treated rats (BD), and brain-dead rats under thoracic epidural blockade (BD-TEB) 180 min after the surgical procedures. The data are presented as the means±SEM; p ANOVA=0.002; *p<0.05 vs. the other groups. B, C, and D, Photomicrographs of mesenteric microvessels in the SH, BD, and BD-TEB groups, respectively.
Table 1 shows the leukocyte-endothelium interaction data obtained from perfused small vessels. Similar results were observed in both the BD and BD-TEB rats, and the number of rolling leukocytes in these two groups was reduced compared to that in the SH group. A reduction in the number of adherent leukocytes in the BD and BD-TEB groups compared to the SH group was also observed. In contrast, the number of migrating leukocytes in both groups of BD rats was higher than that in the SH group. These results were not accompanied by significant changes in the number of WBCs.
The results of the evaluation of the rat mesenteric microcirculation via intravital microscopy.
| SH | BD | BD-TEB | p ANOVA | |
|---|---|---|---|---|
| Rollers | 165 (12)* | 44 (4) | 31 (5) | <0.001 |
| Adherent | 6.9 (0.6)* | 4.5 (0.5) | 3.2 (0.3) | 0.002 |
| Migrated | 1.3 (0.2)** | 2.5 (0.2) | 1.8 (0.2) | 0.01 |
| WBC | 11,771 (1,122) | 10,450 (2,109) | 8,667 (1,196) | 0.3 |
Rollers: leukocytes/5 min; Adhered: leukocytes/100 µm venule length; Migrated: leukocytes/5,000 µm2; WBC: White blood cells/mm3. SH: sham-operated rats; BD: brain-dead non-treated rats; BD-TEB: brain-dead rats under thoracic epidural blockade. These measurements were performed 180 min after the surgical procedures. The data are presented as the means±SEM. *p<0.01 vs. the other groups. **p<0.05 vs. the other groups.
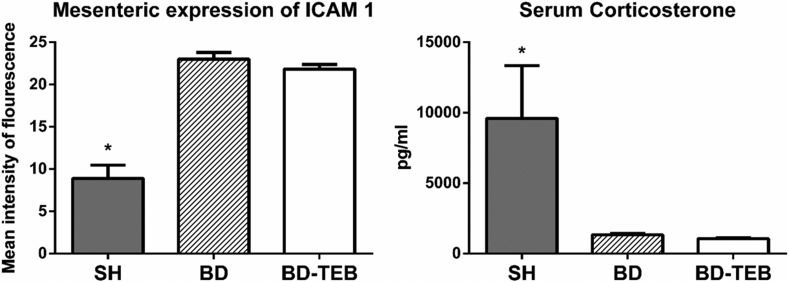
The expression of ICAM-1 was investigated in the mesentery of rats that were not examined via intravital microscopy (Figure 3). The levels of ICAM-1 expression were similar between the BD and BD-TEB groups and exceeded those in the SH group. The serum levels of corticosterone were reduced in the BD and BD-TEB groups compared to the SH group three hours after BD induction (Figure 3).
Expression of intercellular adhesion molecule (ICAM)-1 on mesenteric endothelial cells (p ANOVA <0.001) and the serum levels of corticosterone (p ANOVA=0.01) in sham-operated rats (SH), brain-dead non-treated rats (BD), and brain-dead rats under thoracic epidural blockade (BD-TEB) 180 min after the surgical procedures. The data are presented as the means±SEM. *p<0.05 vs. the other groups.
Similar increases in the serum concentrations of TNF-α, IL-1β, IL-6, and IL-10 were observed in the BD, BD-TEB, and SH groups 180 min after the surgical procedures. The reference values obtained in Naive rats were below the limit of detection of the assay. These results are summarized in Table 2.
The results of the evaluation of the serum cytokine levels via enzyme-linked immunosorbent assays.
| TNF-α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) | IL-10 (pg/mL) | |
|---|---|---|---|---|
| SH | 0.066±0.005 | 0.102±0.017 | 0.131±0.022 | 0.112±0.004 |
| BD | 0.054±0.004 | 0.074±0.004 | 0.130±0.043 | 0.089±0.007 |
| BD-TEB | 0.067±0.007 | 0.090±0.017 | 0.151±0.043 | 0.096±0.010 |
| p ANOVA | 0.4 | 0.9 | 0.2 | 0.2 |
SH: sham-operated rats; BD: brain-dead non-treated rats; BD-TEB: brain-dead rats under thoracic epidural blockade. These measurements were performed 180 min after the surgical procedures. The data are presented as the means±SEM.
In an attempt to attenuate the hemodynamic alterations triggered by BD, thoracic sympathetic blockade was induced in this study via epidural anesthesia with bupivacaine. We found that TEB effectively abolished the hypertensive event observed after BD induction but did not affect the following hypotensive episode. Furthermore, mesenteric hypoperfusion and inflammation, characterized by increased leukocyte migration and ICAM-1 expression, remained unchanged in the BD animals despite TEB. Moreover, the serum levels of cytokines and corticosterone did not change after the treatment of BD rats with TEB.
Hemodynamic instability is a major challenge in the treatment of BD donors because it leads to the impairment of organ perfusion and compromises the viability of these organs for transplantation. Although hypertension may occur during the phase of cerebral herniation, hypotension affects almost all patients after BD 18. Indeed, in animal models, the hypotensive event that accompanies BD is initiated by both rapid and slow augmentation of intracranial pressure 10,19–21. Nevertheless, the important question of how the modification of sympathetic activity after BD impacts microcirculation behavior and hypoperfusion in different organs remains unanswered.
In this study, we observed the hemodynamic, microcirculatory, and inflammatory effects of TEB performed immediately before BD induction in rats that did not receive any drugs for blood pressure maintenance. Thus, our non-treated rats are similar to marginal donors in clinical practice. The chemical sympathectomy produced by TEB using bupivacaine prevented the hypertensive event associated with BD, although a slight increase in mean arterial pressure was observed when the balloon was inflated. Furthermore, we demonstrated for the first time that mesenteric hypoperfusion is not affected by the inhibition of the hypertensive episode. The long episode of hypotension that generally starts immediately after the hypertensive peak was not affected by TEB. This event was associated with rapid and persistent hypoperfusion of the mesenteric microcirculation. Such hypoperfusion was previously demonstrated by our group in the mesentery 8 and by others in the pancreas and the liver 15,16. Additional real-time monitoring of the mesenteric microcirculation during the first 30 min after BD induction confirmed that mesenteric hypoperfusion occurs immediately after BD and concomitantly with the onset of the sustained hypotensive episode (unpublished data). This observation suggests that microcirculatory abnormalities can occur independently of an increase in arterial pressure, suggesting the involvement of other mechanisms triggered by BD in these microcirculatory abnormalities.
It has been demonstrated that BD leads to a decrease in organ perfusion and a progressive activation of endothelial cells and leukocytes 8,22–25. It is known that BD triggers a series of inflammatory effects, including increased expression of adhesion molecules, leukocyte transmigration to the perivascular tissue in various organs, and increased serum levels of cytokines 2,3,26–28. The present data indicate that the inflammation in the mesenteric microcirculation is not affected by TEB. The animals in both BD groups exhibited similar numbers of rolling, adherent, and migrating leukocytes, as well as comparably increased levels of ICAM-1 expression in endothelial cells; these characteristics were associated with a pronounced reduction in the serum corticosterone levels. It is known that BD impairs the endocrine system and decreases the release of hormones such as triiodothyronine, thyroxin, cortisol, and anti-diuretic hormone, suggesting an interruption along the hypothalamic-pituitary-adrenal axis 29,30. In this regard, it has been shown that in healthy subjects, endogenous glucocorticoids that are secreted in large amounts at the early stages of an inflammatory reaction regulate the functions of almost all cellular components of the microcirculation 31. Indeed, the SH rats exhibited increased levels of corticosterone in association with reduced ICAM-1 expression compared to the BD rats. Furthermore, the serum levels of cytokines were similar between the groups, suggesting that the release of cytokines triggered by BD-associated trauma 8,28 was not affected by TEB.
It has been well documented that abnormalities in microcirculatory perfusion, including decreased vascular density, especially in the small vessels, and a large number of non-perfused and intermittently perfused small vessels, which occur in septic patients, are not affected by the global hemodynamic state or the use of adrenergic agents 32,33. The experimental findings are consistent with the clinical evidence that sepsis induces microvascular changes that may play an important role in the development of organ dysfunction 32,34,35. These results justify the attempts to revert these alterations. Alternatively, microcirculatory targets are not currently considered for optimizing the therapy after BD. Fluid replacement, the use of vasoactive drugs, and hormone replacement therapy are the strategies used to achieve hemodynamic stability and to maintain organ function in potential BD organ donors 18. Although these interventions may influence the microcirculation, management aimed at stabilizing the global hemodynamic state does not always ensure the normalization of tissue perfusion, which is an important objective in shock states of various etiologies.
In conclusion, the results of this study indicate that epidural anesthesia with bupivacaine does not affect the sustained hypoperfusion and inflammation in the mesenteric microvessels induced by BD, suggesting that microcirculatory abnormalities can occur independently of changes in mean arterial pressure. These findings clearly demonstrate that therapies aimed at maintaining the microcirculation may effectively compensate for the perfusion abnormalities after BD, thereby potentially improving the quality of donor organs for transplantation.
AUTHOR CONTRIBUTIONSSimas R participated in the research design, performance of the research, the data analysis, and the writing of the paper. Ferreira SG, Menegat L, Zanoni FL, and Correia CJ participated in the performance of the research. Silva IA participated in the research design and the writing of the paper. Sannomiya P and Moreira LF participated in the research design, the data analysis, and the writing of the paper.
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Grant number 09/10759-9.
No potential conflict of interest was reported.