The aim of this study was to characterize Staphylococcus aureus (MRSA) carriage in a dermatology unit.
METHODS:This was a prospective and descriptive study. Over the course of 26 weeks, surveillance cultures were collected weekly from the anterior nares and skin of all patients hospitalized in a 20-bed dermatology unit of a tertiary-care hospital. Samples from healthcare workers (HCWS) were cultured at the beginning and end of the study. Colonized patients were put under contact precautions, and basic infection control measures were enforced. Staphylococcus aureus colonization pressure was determined monthly. Colonized and non-colonized patients were compared, and isolates were evaluated for antimicrobial susceptibility, SCCmec type, virulence factors, and type.
RESULTS:Of the 142 patients evaluated, 64 (45%) were colonized by MRSA (39% hospital acquired; 25% community acquired; 36% indeterminate). Despite isolation precautions, hospital-acquired Staphylococcus aureus occurred in addition to the continuous entry of Staphylococcus aureus from the community. Colonization pressure increased from 13% to 59%, and pemphigus and other bullous diseases were associated with MRSA colonization. Eleven out of 71 HCWs (15%) were Staphylococcus aureus carriers, although only one worker carried a persistent clone. Of the hospital-acquired MRSA cases, 14/28 (50%) were SCCmec type IV (3 PFGE types), 13 were SCCmec type III (46%), and one had an indeterminate type. These types were also present among the community-acquired Staphylococcus aureus isolates. SSCmec type IV isolates were shown to be more susceptible than type III isolates. There were two cases of bloodstream infection, and the pvl and tst virulence genes were absent from all isolates.
CONCLUSIONS:Dermatology patients were colonized by community- and hospital-acquired Staphylococcus aureus. Half of the nosocomial Staphylococcus aureus isolates were SCCmec type IV. Despite the identification of colonized patients and the subsequent contact precautions and room placement, Staphylococcus aureus colonization continued to occur, and colonization pressure increased. Pemphigus and other bullous diseases were associated with Staphylococcus aureus.
Staphylococcus aureus is a versatile pathogen capable of causing a wide variety of infections.1 The prevalence of nosocomial and community-acquired methicillin-resistant Staphylococcus aureus (MRSA) infections has increased in recent years.2 Methicillin resistance of Staphylococcus aureus is mediated by a penicillin-binding protein (PBP2A) that has a low affinity for β-lactam antibiotics and is encoded by the mecA gene.3,4 This gene is carried on a mobile genetic element designated as Staphylococcal Cassette Chromosome mec (SCCmec), which is integrated into the chromosome. Currently, 11 types of SCCmec have been reported.5–7 In healthcare settings, patients who are colonized by or infected with MRSA serve as a reservoir and a source for the spread of this microorganism, which occurs mainly through transiently colonized healthcare workers (HCWs).8,9 Carriage of S. aureus is considered a risk factor for the development of infections, as infections are usually preceded by a period of colonization.10
The objective of this study was to characterize MRSA carriage in a hospital dermatology unit.
METHODSHospital das Clínicas is a tertiary-care teaching hospital that is affiliated with the University of São Paulo, Brazil. It has approximately 2,000 beds and is divided into 6 buildings. The dermatology unit has 20 beds, which are distributed across 10 rooms of one, two, or four beds each. This unit is used for the hospitalization of patients with severe dermatologic conditions. On average, there are 30 admissions per month and 4,500 patient-days each year.
Surveillance cultures of patientsOver a period of 26 weeks that began on May 31, 2005, weekly surveillance cultures for MRSA were obtained from all of the patients admitted to the unit. These samples were collected from the anterior nares and skin lesions. Patients who were positive for MRSA were not followed for further surveillance cultures. Patients who were negative for MRSA were cultured weekly until their discharge from the unit.
Due to the high proportion of patients who tested positive on the first surveillance culture, patients were cultured upon admission and then weekly from the 13th week of the study onward.
The swabs from the anterior nares were collected by introducing a swab into the nasal vestibulum and then rubbing with light pressure. The swabs were transported in sterile tubes containing culture medium and were sent immediately to the laboratory for further processing and analysis. The swabs from skin lesions were collected by gently scraping or rolling the swab across the lesion.
DefinitionsNosocomial acquisition of MRSA was defined as a positive culture in a patient who had been in the hospital for more than 48 hours and whose previous surveillance cultures at both sites had been negative (nares and skin).
For patients who were positive within the first 48 hours of hospitalization, MRSA was considered to have been present at the time of hospital admission. All other colonizations were considered indeterminate as to where MRSA acquisition occurred.
The following patient data were registered upon admission to the study: age, sex, date of admission to the hospital, underlying diseases, hospitalization within the previous 12 months, and use of antimicrobial drugs. The patients were followed until their discharge from the unit. We evaluated the colonization of the patients by dividing the study into six periods.
Control measuresAll patients positive for MRSA were placed in separate rooms or were placed in a cohort with other MRSA carriers and put under contact precautions until hospital discharge or death. Hand hygiene was enforced by requiring the use of alcohol gel. There were two educational meetings with members of the staff in which the control measures and their objectives were discussed. Posters were spread throughout the unit to report the weekly number of patients colonized by MRSA. Patients also received information regarding the contact precautions and their importance.
Surveillance cultures of healthcare workers (HCWs)The anterior nares of the healthcare workers were cultured on two occasions: at the beginning and at the end of the study. HCWs who tested positive were not treated.
EthicsThe study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Commission of the Hospital das Clínicas (approval number: 1072/04).
Analysis of dataContinuous variables are herein presented as the means ± standard deviation or median and range. Frequencies were calculated for the categorical variables. MRSA-colonized patients were compared to non-colonized patients using the χ2 test for categorical variables and the Mann Whitney test for continuous variables. The data were analyzed using Epi Info 6.04 software (CDC, Atlanta, USA). A p-value of 0.05 was considered statistically significant.
For each period, we determined the total number of patient-days and the number of MRSA patient-days (by summing the days that each MRSA-colonized patient remained in the unit after the day that MRSA colonization was first identified). Colonization pressure was also determined for each period by dividing the number of MRSA patient-days by the total number of patient-days (expressed as a percentage).
Microbiological methodsDetection of MRSASamples were plated onto mannitol salt agar (MSA) and inoculated into brain heart infusion (BHI) broth. Mannitol-fermenting colonies were subcultured onto 5% sheep blood agar plates. If the initial MSA culture was negative, a subculture from the BHI broth was carried out on MSA plates to increase the sensitivity of detection. S. aureus was identified by Gram staining and DNAse and catalase tests. Methicillin resistance was determined using Mueller Hinton agar supplemented with 4% NaCl and 6 μg/mL oxacillin according to guidelines of the Clinical Laboratory Standards Institute (CLSI).11 If there was growth of at least one colony-forming unit, the culture was considered positive. S. aureus ATCC 29213 and NCTC 10442 were used as controls.
The phenotypic identification was confirmed by polymerase chain reaction (PCR), amplifying 117 bp and 214 bp fragments from the coagulase and mecA genes, respectively, using the NCL-SA-PS kit (Novo Castra, United Kingdom), as previously described by Kearns et al.12
Susceptibility testingMinimal inhibitory concentrations (MICs) were determined using the broth microdilution method and were interpreted according to CLSI guidelines11,13 for oxacillin, penicillin, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, gentamicin, rifampicin, sulfamethoxazole/trimethoprim, tetracycline and vancomycin.
Molecular typingMolecular typing was performed by digesting whole-cell DNA with the SmaI macrorestriction enzyme (Fermentas Life Sciences, Canada) and then determining the fragment-size patterns obtained with pulsed-field gel electrophoresis (PFGE) using a CHEF DR-II apparatus (Bio-Rad Laboratories, USA).14 The patterns were analyzed as recommended by Tenover et al.15 Types were defined as isolates that differed by at least seven fragments and were identified using letters. Subtypes of a given clonal type were defined as those isolates that differed by fewer than seven fragments and were identified using numbers.
Multilocus sequence typing was performed for 10 isolates according to methods described elsewhere.16 This sequence typing was performed for one isolate of each PFGE type (seven isolates), one isolate for which the SCCmec type could not be determined and two isolates in which mecA could not be detected.
SCCmec typingThe determination of the SCCmec type was performed using the multiplex PCR method, as described by Oliveira & Lencastre.17 DNA was extracted using a commercial kit (Genomic Prep Cells and Tissue DNA Isolation, Amersham Pharmacia, Biotech, Germany). PCR amplifications were performed using a GeneAmp PCR System 2400 thermocycler (Perkin-Elmer, Waltham, MA, USA). The MRSA strains NTCT 10442, N315, 85/2082 and JSCS 1968, which belong to SCCmec types I, II, III, and IV, respectively, were used as positive controls.
Detection of genes for virulence factorsOne isolate from each PFGE subtype was evaluated for the Panton Valentine leukocidin (pvl), LukE-LukD leukocidin (lukE-lukD) and toxic shock syndrome toxin-1 (tst) virulence genes by PCR using primers described elsewhere.18 Briefly, samples were denatured for 5 minutes at 94°C; subjected to 30 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 1 minute, and extension at 72°C for 2 min; and then subjected to a final extension at 72°C for 5 minutes. The MR108 (positive for pvl) and N315 (positive for lukE-lukD and tst) isolates were used as controls.
RESULTSDuring the study period, 153 patients were admitted to the dermatology unit. Of these, 11 were lost to follow-up, and 142 were evaluated until discharge and included in the analysis.
Of the 142 patients, 64 (45%) were colonized by MRSA. There were 26 patients who were positive only from the culture of the anterior nares (one patient harbored two isolates), 11 who were positive only from skin culture and 27 who had tested positive from both sites (two patients each harbored three isolates). Thus, 94 MRSA isolates from 64 patients were microbiologically evaluated.
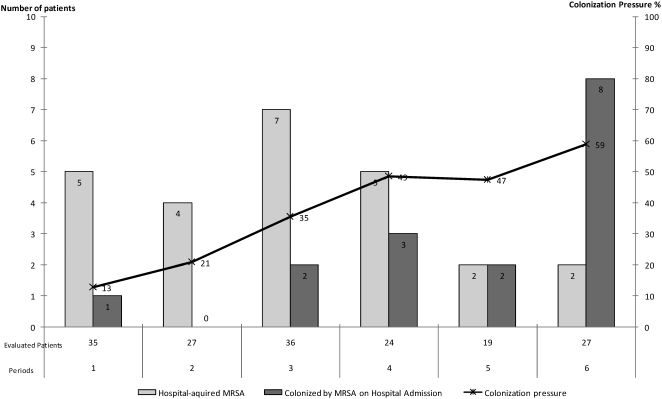
Among the 64 patients (45%) who were colonized by MRSA, 25 (39%) had hospital-acquired infections, 16 (25%) were colonized at the time of admission and 23 patients (36%) had an indeterminate site of acquisition. The distributions over time of patients who were colonized at the time of admission and of those who acquired MRSA in the hospital are presented in Figure 1. Despite the control measures, the nosocomial acquisition of MRSA occurred during the entire study period. In addition, during the entire study period, there was a continuous entry of patients who were MRSA positive at the time of admission, and the colonization pressure also increased over the six-month study period (Figure 1).
The characteristics of the MRSA-colonized and non-colonized patient populations are presented in Table 1. Bullous diseases, such as pemphigus and others, were significantly more frequent among colonized patients.
Characteristics of MRSA-colonized patients and non-colonized patients in the dermatology unit over a 6-month period.
| Characteristics | Colonized (n = 64) | Non-colonized (n = 78) | p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Male sex | 28 (44%) | 37 (47%) | 0.66 |
| Age (years)-mean (SD)-median (range) | 42.8 (21.8)42 (1-84) | 38.8 (25.7)35 (0-92) | 0.33 |
| Total days of hospitalization-mean (SD)-median (range) | 24.3 (20.8)17 (2-118) | 15.4 (24.4)8.5 (1-168) | 0.02 |
| Days of hospitalization until MRSA detection-mean (SD)-median (range) | 9.5 (10.4)6 (0-49) | NANA | |
| Hospitalization within the previous 12 months-in our hospital | 30 (47%)18 (60%) | 25% (32%)15 (60%) | 0.070.21 |
| Previous use of antimicrobial drugs | 38 (59%) | 36 (46%) | 0.12 |
| Underlying diseases | |||
| Bullous diseases | 22 (34%) | 6 (8%) | <0.001 |
| Pemphigus | 15 (23%) | 3 (4%) | <0.001 |
| Other bullous | 7 (11%) | 3 (4%) | 0.10 |
| Atopy | 11 (17%) | 10 (13%) | 0.47 |
| Cancer | 5 (8%) | 9 (12%) | 0.46 |
| Erythrodermia | 8 (13%) | 6 (8%) | 0.34 |
| Infectious | 7 (11%) | 8 (10%) | 0.90 |
| Auto-immune | 4 (6%) | 8 (10%) | 0.10 |
| Psoriasis | 6 (9%) | 8 (10%) | 0.86 |
| Eczema | - | 7 (9%) | 0.14 |
| Death during hospitalization | 4 (6%) | 1 (1%) | 0.11 |
SD: standard deviation NA: not applicable.
The SCCmec types and molecular types of the 94 isolates were determined. When a patient harbored more than one isolate of the same SCCmec type and PFGE type, only one isolate for that patient was included in the final analysis. Therefore, 67 isolates from 64 patients were analyzed. There were seven molecular PFGE types (A to G) that were divided into 30 subtypes. The distribution of the patient isolates according to the origin of acquisition, SCCmec type and molecular type is presented in Table 2. The results of the MLST for isolates of each PFGE type (A to G) are presented in Table 3.
SCCmec type and molecular type of 67 MRSA isolates according to the origin of acquisition.
| Origin of the MRSA (n) | SCCmec type (n) | Molecular PFGE type (n) |
|---|---|---|
| Hospital acquired (n: 28) | III (13) | E (9) |
| F (2) | ||
| G (2) | ||
| IV (14) | B (6) | |
| A (5) | ||
| C (3) | ||
| ND (1) | C (1) | |
| Positive at hospital admission (n:16) | III (4) | E (4) |
| IV (11) | A (7) | |
| C (4) | ||
| ND (1) | C (1) |
MRSA: methicillin-resistant S. aureus; PFGE: pulsed-field gel electrophoresis; ND: not determined.
Multilocus sequence typing results for 10 methicillin-resistant S. aureus subtypes that colonized patients hospitalized in the dermatology unit over a period of 6 months.
| Subtype determined by PFGE | Sequence type | SCCmec type |
|---|---|---|
| A1 | 1176 | IV |
| B1 | 8 | IV |
| C1 | 97 | IV |
| C6 | 5 | ND |
| D | 8 | IV |
| E1 | 239 | III |
| E8 | 239 | - |
| E9 | 239 | - |
| F | 239 | III |
| G | 239 | III |
PFGE: pulsed-field gel electrophoresis; SCCmec: staphylococcal chromosomal cassette mec; ND: not determined; -: absence of mecA.
The SCCmec type IV isolates belonged to four different PFGE molecular types (A-D) and four different sequence types (ST5, ST8, ST97 and ST1176). However, the PFGE type and ST did not correlate completely, as ST8 belonged to two different PFGE types (B and D), and there were 2 ST (ST97 and ST5) among the isolates of PFGE type C.
Of the SCCmec type III isolates, there were 3 PFGE molecular types (E-G), all of which belonged to ST239, as does the multiresistant Brazilian Endemic Clone (BEC).
Of the isolates obtained at the time of patient admission, most were SCCmec type IV, but there were also four isolates that were similar to the BEC.
The hospital-acquired isolates were evenly distributed among SCCmec types IV and III.
Among the 30 isolates tested (all carried SCCmec type IV), the pvl and tst virulence factors were absent from all of them, and the lukD-lukE gene was present in all of them.
During the study period, there were two cases of bloodstream infections. These were caused by MRSA of SCCmec type IV and PFGE type A. These patients had been colonized by the same subtypes at earlier time points (25 and 40 days prior).
Thirty-seven healthcare workers (HCWs) were cultured at the beginning of the study, and 34 were cultured at the end of the study. Of these, five (14%) were positive on the first occasion, and six (18%) were positive on the second. The distribution of the isolates from healthcare workers according to SCCmec type and molecular type is presented in Table 4. Fifteen HCWs were cultured on both occasions, and three were positive on both occasions, but only one healthcare worker (belonging to the housekeeping staff) presented the same subtype twice (C3). However, none of the patients presented the C3 subtype.
The SCCmec and molecular types of 11 MRSA isolates obtained from healthcare workers at the beginning and the end of the study.
| Healthcare workers | SCCmec type (n) | Molecular PFGE type (n) |
|---|---|---|
| Beginning of study (n: 37) 5 (14%) positive for MRSA | IV (5) | C (3)B (1)A (1) |
| End of study (n: 34) 6 (18%) positive for MRSA | IV (5)III (1) | A (1)B (3)C (1)E (1) |
MRSA: methicillin-resistant S. aureus; PFGE: pulsed-field gel electrophoresis.
The antimicrobial susceptibilities of the isolates that colonized 60 patients and 11 healthcare workers are shown in Table 5.
Antimicrobial susceptibility according to SCCmec type of 71 MRSA isolates that colonized 60 patients and 11 healthcare workers in the dermatology unit over a period of 6 months.
| Antimicrobial drug | Number of susceptible isolates (%) | ||
|---|---|---|---|
| SCCmec type III (n: 25) | SCCmec type IV (n: 46) | Total (n: 71) | |
| Chloramphenicol | 20 (80%) | 46 (100%) | 66 (93%) |
| Ciprofloxacin | 0 | 43 (93%) | 43(61%) |
| Clindamycin | 0 | 35 (76%) | 35 (49%) |
| Erythromycin | 0 | 15 (33%) | 15 (21%) |
| Gentamicin | 0 | 27 (59%) | 27(38%) |
| Rifampicin | 9 (36%) | 46 (100%) | 55 (77%) |
| Sulfamethoxazole/ trimethoprim | 0 | 45 (98%) | 45 (63%) |
| Tetracycline | 0 | 40 (87%) | 40 (56%) |
| Vancomycin | 25 (100%) | 46 (100%) | 71 (100%) |
Colonization with MRSA was prevalent among patients hospitalized for dermatological diseases (45%), and this colonization was due to both hospital acquisition and colonization prior to admission.
This study began by examining routine surveillance cultures to determine the extent of the problem and to identify patients who should be placed under contact precautions, such as placement in a private room or in a patient cohort. Through the enforcement of these measures, we expected to halt the nosocomial transmission of MRSA. Community-acquired MRSA (CA-MRSA) has been rarely described in Brazil and has been shown to occur mainly in the southern region of the country19,20 near the border with Uruguay, which is a country in which CA-MRSA is a significant problem. In addition, in a previous study of MRSA isolates obtained from bloodstream infections,21 there were six cases of hospital-acquired MRSA bacteremia the dermatology unit. Thus, we expected most of the cases of MRSA colonization to be hospital acquired. However, in our dermatology unit, we observed that approximately half of the colonization cases were hospital-acquired and that a significant proportion of patients were positive for MRSA at the time of admission. Our results suggest that many patients are colonized by community-acquired MRSA or are colonized at other healthcare facilities and may be a source of MRSA transmission to other patients in the hospital. There was a significantly higher proportion of patients with pemphigus and other bullous diseases among MRSA carriers, which suggested that disseminated bullous disease is a risk factor for MRSA colonization.
During the entire study period, nosocomial transmission of MRSA occurred as well as admission of colonized patients. This likely generated or increased colonization pressure and made control more difficult despite the efforts directed to prevent nosocomial MRSA transmission. Over the six-month study period, the frequency of nosocomial-acquired cases tended to decrease, but the colonization pressure increased from 13% to 59%. We attributed this decrease in the nosocomial acquisition of MRSA to the extensive efforts of the staff, which included improving the isolation conditions and the infection control measures. Our original goal was the complete eradication of nosocomial transmission, but this goal was not achieved. The colonization pressure was first evaluated for vancomycin-resistant enterococci.22 This type of resistance has been studied in MRSA and has been shown to play a role as a risk factor for nosocomial transmission.23 In one study, a colonization pressure above 30% led to a 5-fold increase in the risk of MRSA colonization.24
Due to the large number of patients who are colonized at the time of admission, the use of routine surveillance cultures and the placement of colonized patients into cohorts may not affect the rates of colonization unless all patients are immediately placed under contact precautions even before the surveillance cultures are analyzed. Furthermore, the success of MRSA control that is based mainly on the identification of carriers through the analysis of surveillance cultures depends on the sensitivity of these cultures, which may not be sufficiently high to detect all colonized patients. Some authors have reported that the lack of throat culturing may lead to an underestimation of the percentage of MRSA carriers by as much as 8 to 18%.25,26
The clinical impact and the relevance of routine patient culturing may be contested because, despite a high proportion of colonized patients, there were only two cases of bloodstream infection in our six-month study period. Routine culturing and the use of patient cohorts and private rooms for positive patients are expensive and labor-intensive measures. In our hospital, most rooms are meant to hold two or four patients, and there are very few private rooms. During patient isolation, the rooms are not used at full capacity, which is a problem because there is a nationwide shortage of hospital beds. In addition, although the carriage of S. aureus is considered a risk factor for the development of infection,10 one prospective study found that nasal colonization with MRSA was a poor predictor of the subsequent occurrence of MRSA respiratory tract or bloodstream infection.27
This study also addressed the role of HCWs in the transmission of MRSA. Among HCWs, MRSA was observed in 15% of the cultures. Only three HCWs were positive on both occasions, but two HCWs harbored different molecular types of MRSA at each culture, suggesting transient carriage. Interestingly, half of the HCWs who were positive for MRSA harbored isolates of subtypes that were not present among the patients. This result suggests that a large proportion of the HCW colonizations were acquired outside of the hospital, either in the community or at other institutions.
MRSA with SCCmec type III was multidrug resistant and belonged to the Brazilian Endemic Clone (BEC). Molecular typing by pulsed-field gel electrophoresis (PFGE) revealed three different types and one predominant type (79%). Within this predominant type, there were nine different subtypes. The BEC has been endemic in Brazil for more than a decade.28 During this time, it is likely that changes occurred that made Tenover's criteria17 inadequate to interpret its clonality. In this situation, multilocus sequence typing (MLST) is more useful because it evaluates seven housekeeping genes that are more stable over time.29 BEC presents as sequence type ST-239,28 and all of the SCCmec type III isolates tested in our study also belonged to this ST.
The presence of MRSA with SCCmec type IV at our hospital is a more recent phenomenon than the existence of the BEC. Therefore, the PFGE typing reflected clonality that was similar although not identical to the MLST.21 Furthermore, we expected that the SCCmec type IV would be mainly community acquired. However, similar to other geographic areas,30 it seems as though this type has crossed hospital borders and circulates not only in the community but also in the hospital environment.
As observed in other studies conducted at our hospital,21,31 SCCmec type IV MRSA isolates from dermatologic patients did not express the Panton-Valentine leukocidin (PVL) virulence factor. The tst gene was also absent from all of the isolates. This finding is interesting because PVL-producing clones have been associated with skin and soft tissue infections32,33 and because TSST-1 is the cause of skin lesions. On the other hand, in a hospital environment, where patients are severely ill and have many diseases, a virulent strain of S. aureus would probably not be successful because it would cause severe infection and high mortality, as it does in the community.
Our study has limitations. First, it was our original intention to use the CDC definitions, but the necessary information was not available for a large proportion of the included patients. Because of this lack of information, we had to introduce the “indeterminate” category. There was a large proportion of patients for which the origin of colonization was deemed indeterminate, which was in part due to the fact that surveillance culturing immediately upon admission was only initiated halfway into the study. In addition, we did not observe SCCmec types other than types I to IV, and there were a few isolates for which we could not determine the type. Finally, it was not possible to perform MLST analyses on each of the PFGE subtypes.
In summary, almost half of the dermatology patients in this study were colonized by MRSA at the time of hospital admission or acquired MRSA while in the hospital. Half of the nosocomial MRSA cases were SCCmec type IV. Nosocomial MRSA colonization continued throughout the study period despite the identification of colonized patients, the use of contact precautions and patient cohorting or the placement of patients in private rooms based on their surveillance cultures. Colonization pressure continuously increased during the study period, which may partly explain the difficulty in controlling the spread of MRSA. Pemphigus and other bullous diseases were significantly associated with MRSA colonization. The healthcare workers were predominantly colonized by SCCmec type IV MRSA, and this colonization was found to be transient and predominantly community acquired.
This study was funded by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)- 06/03003-7; 05/5754-0 and 06/00569-0.
We thank Robson E. Soares for his suggestions in the laboratory and with the manuscript.
No potential conflict of interest was reported.
Pacheco RL was responsible for the collection of patient data and clinical specimens, laboratory procedures and analysis of data. Lobo RD was responsible for the collection of data and specimens, implementation of control measures and contributed to the writing of the manuscript. Oliveira MS was responsible for the collection of data and specimens, implementation of control measures and contributed to the writing of the manuscript. Farina EF was responsible for the laboratory procedures and analysis of data. Santos CR was responsible for the collection of data and specimens and implementation of control measures. Costa SF supervised the laboratory work. Padoveze MC and Garcia CP contributed to the writing of the manuscript. Trindade PA was responsible for the laboratory procedures. Quitério LM and Rivitti EA were responsible for the organization of the unit and implementation of control measures. Mamizuka EM was responsible for the laboratory procedures. Levin AS was responsible for the general supervision and the writing of the manuscript.