Oral lesions are among the early signs of HIV infection and can predict its progression to acquired immunodeficiency syndrome (AIDS). A better understanding of the oral manifestations of AIDS in both adults and children has implications for all health care professionals. The knowledge of such alterations would allow for early recognition of HIV-infected patients. The present paper reviews epidemiology, relevant aspects of HIV infection related to the mouth in both adults and children, as well as current trends in antiretroviral therapy and its connection with orofacial manifestations related to AIDS.
HIV infection remains a significant health care problem.1 Since Barre-Sonoussi and Gallo’s initial description of the human immunodeficiency virus type I (HIV-1) in 1983 and Clavel et al. first described HIV-2 in 1986, these two viruses have been recognized for almost 20 years as the primary cause of the acquired immunodeficiency syndrome (AIDS).2
By the end of 2005, an estimated 40.3 million people were alive with HIV infection in the world, the vast majority of whom were resident in low-income countries.3 However, in 2007, advances in the methodology of estimating HIV epidemics applied to an expanded range of country data, resulting in substantial changes to the estimates of the number of persons living with HIV worldwide; nevertheless, the qualitative interpretation of the severity and implications of the pandemic have altered little.4 The estimated number of persons living with HIV worldwide in 2007 is now assumed to be 33.2 million [30.6–36.1 million], a reduction of 16% compared with the estimate published in 2006 (39.5 million [34.7–47.1 million]).4 Of this number, only 1.6 million live in high-income countries; the remaining more than 95% of HIV-infected people live in developing countries.5 In developed countries, the number of children newly infected with HIV has decreased dramatically. However, in developing countries, there are an estimated 3.5 million children younger than the age of 15 who are infected. In developing countries in 2007, an estimated 330,000 children younger than the 15 years of age died of AIDS, and more children younger than the age of 5 years die from AIDS now than from any other cause.6
The prevalence and incidence of HIV/AIDS vary considerably from continent to continent, from country to country, and from region to region. Several countries in sub-Saharan Africa report infection rates of 30%, especially in urban areas; however, in other countries, HIV prevalence still remains low. Low national prevalence rates can be misleading. They often disguise serious epidemics that are initially concentrated in certain localities or among specific population groups and that threaten to spill over into the wider population.7
HIV infection leading to AIDS has been a major cause of illness and death among children, teens, and young adults worldwide. AIDS has been the sixth leading cause of death in the United States among 15- to 24-year-olds since 1991. In recent years, AIDS infection rates have been increasing rapidly among teens and young adults. Half of all new HIV infections in the United States occur in people under 25; thousands of teens acquire new HIV infections each year.8 In 2007 alone, 420,000 infants and children were newly infected with HIV in developing countries, more than 1,150 every day. An estimated 330,000 children died from HIV and AIDS during 2007, joining more than 4 million children already claimed by the epidemic. 6
There are several ways in which someone can become infected with HIV, and some of these transmission routes are well defined. HIV infection can be transmitted through unprotected sexual intercourse with an infected partner The HIV virus can be transmitted through unprotected oral sex, both from fellatio and cunnilingus, although the precise degree of risk of disease transmission to and from the mouth is difficult to establish as these practices often take place along with insertive sexual intercourse.9 Injection or transfusion of contaminated blood or blood products (infection through artificial insemination, skin grafts, and organ transplants is also possible),10 sharing unsterilized injection equipment that was previously used by an infected person,11 and maternal-fetal transmission (during pregnancy, at birth, and through breastfeeding)12,13 are other transmission routes.
Occupational HIV infections of healthcare or laboratory workers may occur, but this mode of infection is not frequent.14 Transmission of HIV from an infected patient to a health-care worker has been documented after parenteral or mucous-membrane exposure to blood. However, this risk is less than 1%, is limited to exposure to blood, and can be further minimized through the availability of more effective antiretroviral therapy (ART).15
There remains little evidence that HIV is transmitted via oral fluids.16 However, saliva seems to play an important role in an individual’s protection from HIV infections. The saliva of non-HIV-infected persons contains non-immune endogenous inhibitors of HIV such as mucins, defensins, thrombospondin, and various salivary proteins, in particular the secretory leukocyte protease inhibitor.17 There is also evidence that the hypotonicity of saliva itself exerts a significant inhibitory effect on cell-associated HIV replication.18
The risk of transmission of HIV from a patient to a dental health care worker remains very low, if not infinitesimal.19 Transmission of HIV from an infected dental health care worker is also rare, although possible.20 Nevertheless, dental health care workers are at risk of nosocomial acquisition of HIV and other blood-borne viruses (BBVs), and these individuals should be aware of, and follow available national guidelines on occupational exposure to BBVs.21
As in other virus infections, the individual course of HIV infection depends on both host and viral factors; however, the factors that may predispose one to or promote the development of the AIDS syndrome are largely unknown.23 The clinical course of AIDS described in the following sentences refers to HIV infection in the absence of highly active antiretroviral therapy (HAART). Several factors, including immunological and virological variables, have been reported to predict disease progression.24 The acute viral syndrome of primary HIV infection (which is defined as the time period from initial infection with HIV to the development of an antibody response) shows symptoms that often resemble those of mononucleosis.25 These symptoms appear within days to weeks of exposure to HIV. However, clinical signs and symptoms may not occur in all patients.26
After the acute infection, equilibrium between viral replication and the host immune response is usually reached, and many infected individuals may have no clinical manifestations of HIV infection for years. Even in the absence of antiretroviral treatment, this period of clinical latency may last 8–10 years or more.27 However, the term “latency period” may be misleading, given the incredibly high turnover of the virus and the relentless daily destruction of CD4+ T-cells.28 At the end of the latency period, a number of symptoms or illnesses may appear that do not fulfill the definition of AIDS. These symptoms include slight immunological, dermatological, hematological, neurological, and orofacial signs.29
Oral manifestations are among the earliest and most important indicators of HIV infection.30 At present, three groups of oral manifestations of AIDS are defined based on their intensity and features. Group 1 is composed of seven cardinal lesions (oral candidosis, hairy leukoplakia, Kaposi sarcoma, linear gingival erythema, necrotizing ulcerative gingivitis, necrotizing ulcerative periodontitis, and non-Hodgkin lymphoma) that are strongly associated with HIV infection.31 The second group includes atypical ulcers, salivary glands diseases, viral infection such as cytomegalovírus (CMV), herpes simplex virus (HSV), papillomavirus (HPV), and herpes zoster virus (HZV). On group 3 are lesion rarer than those on groups 1 and 2, such as diffuse osteomyelitis and squamous cell carcinoma.32 The presence of oral lesions can have a significant impact on health-related quality of life. Oral health is strongly associated with physical and mental health, and there are significant increases in oral health needs in people with HIV infection, especially in children, and in adults particularly in relation to periodontal diseases. Thus, physical and mental health measures of HIV patients should incorporate indicators of oral functioning and well-being. Data obtained in the Coutler et al. study have shown that a one-point increase in oral health was associated with a 0.05 (p < 0.000) increase in mental health and a 0.02 increase in physical health (p = 0.031).33
ADVANCEMENT IN THE TREATMENT OF HIV DISEASESignificant advancement has been made in the treatment of HIV disease. This progress has contributed to a decrease in AIDS-related deaths and, consequently, has increased the number of individuals living with HIV throughout the world.34 The treatment of HIV is directed principally towards the specific inhibition of HIV replication (antiretroviral therapy) and the prevention and management of opportunistic infections and malignancies;35 therefore, the ultimate goal of antiretroviral therapy should always be borne in mind: to prolong the patient’s life while maintaining the best possible quality of health and life.36
Antiretroviral drugs (ARDs) now comprise four classes of agents: nucleoside analog reverse transcriptase inhibitors (NRTIs), non-nucleoside analog reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and entry inhibitors.37 This last class of drug blocks HIV entry into host cells and has only become clinically available recently; these drugs include attachment inhibitors, co-receptor inhibitors, and fusion inhibitors, and they are usually reserved for treating HIV-1 infection in treatment-experienced patients.38
Combination therapy, using three or more drugs (usually, but not always, two NRTIs and either a PI or NNRTI) reduces HIV viremia to below detectable levels.39 Such combination therapy, known better as antiretroviral therapy (ART) or highly active antiretroviral therapy (HAART), has greatly improved the prognosis for persons infected with HIV. However, as with almost all antimicrobials, in vivo resistance to NRTIs, NNRTIs, and PIs has been documented as well as transmission of antiretroviral resistant strains of HIV.40 In addition, the various ARDs can give rise to a wide spectrum of orofacial adverse reactions, such as erythema multiforme, hyperpigmentation, ulcers, cheilitis, lipodystrophy syndrome, and so on; the relationships of these reactions to the drugs used are summarized in Table 1. HIV-associated lipodystrophy syndrome is considered to be one of the most clinically relevant adverse effects of ART.41 This syndrome is characterized by a generalized loss of fatty tissue.42 The nasolabial regions and temples are the most common sites of facial involvement, although orbital fat can also be lost when fatty tissue loss is severe. Fat wasting in the limbs leads to prominence of the subcutaneous veins while that of the face and buttocks leads to marked hollowing and wrinkling of the skin.43 Effective ARTs are rarely available to HIV-infected persons in the developing world, particularly in areas of Africa. In some countries that have no specific HIV therapy, the treatment of HIV disease is still principally directed toward the elimination of opportunistic infections, and even this treatment is often unavailable.44 In the absence of specific HIV therapy, health care resources are being increasingly utilized but with little survival benefit for the individual. Resources available for treating patients vary considerably between the richer and poorer countries of the continent.45 For example fluconazole only recently became widely available in South Africa, and even topical antiseptic agents, such as gentian violet which are used in Uganda to treat trigeminal zoster infections, are of limited availability. Extracts of traditionally used East African medicinal plants (e.g., Entada abyssinica, Terminalia spinosa, Harrisonia abyssinica, Ximenia caffra, Azadirachta indica, and Zahna Africana) that have in vitro antifungal actions have been advocated as possible therapeutic alternatives to more expensive antifungal agents.46 Likewise, 0.5% lawsone methyl ether preparations with in vitro antifungal activity similar to that of chlorhexidine gluconate and 1.0% clotrimazole cream have been suggested for the treatment of candidal infections in the developing world.47
Oral and systemic side effects of antiretroviral drugs (ARDs)
| Oral side effect | Class of drug | Name of drug |
|---|---|---|
| Erythema multiforme | NRTIs | Zidovudine (ZVD)Abacavir (ABC)Didanosine (DDI)Zalcitabine |
| NNRTIs | EfavirenzDelaviridineNevirapineSaquinavir | |
| Hyperpigmentation | NRTIs | Zidovudine (ZVD) |
| Lipodystrophy | NRTIs | Stavudine |
| PIs | SaquinavirRitonavir | |
| Xerostomia | NRTIs | Lamivudine (3TC)Didanosine |
| PIs | SaquinavirIndinavirNelfinavirRitonavir | |
| Parotid lipomatosis | PIs | IndinavirRitonavirSaquinavirNelfinavirAmprenavir |
| Cheilitis | PI | Indinavir |
| Perioral paresthesia | PIs | RitonavirAmprenavir |
| Taste disturbances | PIs | IndinabirRitonavir |
| Facial edema | PIs | Ritonavir |
| Ulcers | NRTIs | AbacavirZalcitabineNevirapine |
| Lip enlargement | EIs | Enfuvirtide |
NRTI = nucleoside analog reverse transcriptase inhibitors; NNRTI = non-nucleoside analog reverse transcriptase inhibitors; PIs = protease inhibitors; EI = entry inhibitors
Antiretroviral therapy is very effective at suppressing viral replication. However, viral rebound with resistance does occur, primarily due to sub-optimal compliance and drug toxicities. It is during the period of failing HAART when the HIV load may well be high or rising that the transmission of drug-resistant HIV to susceptible individuals occurs.48 The aforementioned trends in anti-HIV therapy are of relevance to general dental practice. People living with HIV infection may complain of, or have, orofacial features of ART. In contrast, HIV-infected individuals taking ART are likely to have the well-recognized, common orofacial features of untreated HIV disease or may develop others oral manifestations related to ART.49
CURRENT ASPECTS OF THE ORAL MANIFESTATIONS OF AIDSSince the advent of HAART, clinical and epidemiological observations have shown a considerable decline in the mortality and morbidity of HIV-positive patients, which can be attributed to a reduction of HIV viral load and the recovery of immune function in previously ill subjects.50 Patients are protected to some extent against several oral lesion, i.e., candidosis, salivary gland disease, sarcoma, Kaposi’s sarcoma, and oral hairy leukoplakia.51 The prevalence of all oral lesions has decreased by more than 30% since the introduction of HAART. 52 For example, in a study conducted by Tukutuku et al.53, the prevalence of necrotizing ulcerative gingivitis (NUG) and periodontitis (NUP) before HAART was 17%, and 16% of all lesions included these bacterial infections; nowadays the rates are lower at 10% for NUG and 5% for NUP.54 However, the prevalence of some oral lesions has nevertheless increased, such as HIV salivary gland disease, or remained the same, such as oral candidosis, aphtous ulcers, and Kaposi’s sarcoma.55
The oral manifestations of AIDS in adults (Table 2) and children (Table 3) were classified before the advent of ART by EC-Clearinghouse.56–58 Although the EC-Clearinghouse classification was developed over a decade ago, there have been few new infections recognized in HIV-infected persons, perhaps reflecting the wide availability of HAART in the developed world.1
Classification of the oral manifestations of HIV disease in adults
| Group 1lesions strongly associated with HIV infection | Group 2lesions less commonly associated with HIV infection | Group 3lesions seen in HIV infection |
|---|---|---|
Candidosis
| Bacterial infections
| Bacterial infections
|
| Hairy leukoplakia | Melanotic hyperpigmentation | Cat-scratch disease |
| Kaposi’s sarcoma | Necrotizing (ulcerative) stomatitis (Figure 5) | Drug-reactions
|
| Non-Hodgkin’s lymphoma | Salivary gland diseases
| Epithelioid (bacillary) angiomatosis |
Periodontal disease
| Thrombocytopenic purpura | Fungal infections other than candida
|
| Ulceration NOS (not otherwise specified) | Neurological disturbances
| |
Viral infections
| Viral infections
|
Classification of oral manifestations of pediatric HIV disease
| Group 1lesions commonly associated with pediatric HIV infection | Group 2lesions less commonly associated with pediatric HIV infection | Group 3lesions strongly associated with HIV infection but rare in children |
|---|---|---|
Candidosis
| Seborrhoeic dermatitis | Neoplasms
|
| Herpes simplex virus infection | Bacterial infections of oral tissues
| Oral hairy leukoplakia |
| Linear gingival erythema | Periodontal diseases
| Tuberculosis-related ulcers |
| Parotid enlargement | Viral infections
| |
Recurrent aphthous ulcers
| Xerostomia |
The frequency and presentation of some oral lesions associated with HIV infection will, and do, vary with the geography. Patients who do not receive ART are likely to still have the common oral features of HIV disease: candidosis (typically acute pseudomembranous candidosis), hairy leukoplakia, Kaposi’s sarcoma, and perhaps periodontal disease.59 Tuberculosis is more likely in persons residing in or migrating from the developing world, while periodontal disease and other oral lesions due to the use of antiretroviral drugs seem to be most commonly reported in individuals in the developed world.60–62
Fungal InfectionsOral CandidosisCandida albicans is the predominant yeast that colonizes the oral cavity of both healthy subjects and HIV-infected individuals in the developed and developing world.63 However, oral pseudomembranous candidosis (Figure 1) still remains the most common fungal infection of HIV disease; it has been associated with more frequent progression of HIV to AIDS and has been also used as a clinical marker to define the severity of HIV infection,64 with pseudomembranous candidosis usually followed by erythematous candidosis.
Candidal infection has been reported in adults, with a prevalence varying from 1.5 to 56%,65 with the higher prevalence rates in the developing world.65 The wide variability in the prevalence of oral candidosis may be attributed to a variety of factors including the socio-demographic and clinical characteristics of the study group and the diagnostic methods employed.47 Pseudomembraneous candidosis (PC) is the most common clinical presentation of all candidal infections (ranging from 55.8 to 69.7%), followed by erythematous candidosis (EC) (25.7–50%), angular cheilitis (13.7–27.1%), and hyperplastic candidosis (0–1.7%).66,67
Among children in the developed and developing world, rates of oral candidosis have been described as varying from 22.5 to 83.3%.68 PC infection seems to be the most prevalent form in children69 followed by EC,70 and then angular cheilitis as the third most prevalent.71 However, EC has been occasionally reported to be more prevalent than PC.72
The improvement in the immune system of patients receiving HAART may explain the reduction in the prevalence of this opportunistic infection in these groups of HIV-infected individuals. The frequency of oral candidosis usually correlates with a falling CD4+ T lymphocyte count and a rising HIV viral load.73
Other factors that have been implicated in the predisposition of HIV patients to oral candidal infection are age under 35 years, injection drug use, and smoking more than 20 cigarettes a day.47 In contrast, some recent studies have not revealed any specific features that may predispose these patients to oral candidosis.74
Viral InfectionsOral hairy leukoplakiaOral hairy leukoplakia (OHL) is a clinical manifestation of Epstein-Barr virus (EBV) infection almost exclusively found in patients with untreated advanced HIV disease and typically occurs on the lateral border of the tongue of HIV–infected individuals and other groups of immunocompromised individuals (Figure 2).75 The prevalence of OHL in recent studies of HIV-infected adults varies from 0.42 to 38% in both developed and developing countries.76–78 The increased prevalence of OHL might be related to a higher exposure to EBV,79 a lower CD4+ count, and a higher HIV viral load.80
Because OHL is asymptomatic and has no malignant potential, it rarely requires treatment. Nevertheless, acyclovir, and more recently valacyclovir, has been used for the treatment of OHL; unfortunately, acyclovir resistance can prevent the clinical resolution of OHL.81 As discussed previously, most but not all, relevant studies have observed that the frequency of OHL falls with HAART, thus further adding to the rationale that there is little if any need for active intervention in OHL.82
Oral Kaposi’s sarcomaKaposi’s sarcoma (KS) is a malignant, multifocal systemic disease that originates from the vascular endothelium and has a variable clinical course. KS is caused by human herpes virus 8 (HHV-8), which is transmitted sexually or via blood or saliva.83 The most frequently involved site is the skin, but mucous membranes, the lymphatic system, and viscera, in particular the lung and gastrointestinal tract, can also be involved.84 In patients with HIV disease, KS usually arises when the CD4+ T cell count is less than 200.85
The prevalence of oral Kaposi’s sarcoma of the mouth varies from 0 to 12% in Africa and 0 to 38% in US and Europe. However, differences in the frequency of both oral and non-oral KS in HIV disease between the developed and developing world are likely to exist.86 In the developed world, the incidence of HIV-related KS began to decline from 25.6 cases per 1000 person-years (95% confidence interval [CI], 21.8–29.9) in the early 1990s to an average incidence of 7.5 per 1000 person-years (95% CI, 3.4–16.7) in 1996 and 199787 before HAART became available; this trend became more pronounced thereafter. In contrast, the prevalence of KS has risen alarmingly during this same time period in Africa.88 Since the advent of AIDS, KS has become more frequent in both genders, and the male to female ratio has changed from 19:1 to 1.7:1, particularly in East Africa. Recently, the high prevalence of oral KS was demonstrated by the observation that 18.6% of a group of HIV-infected patients in Zimbabwe and from 6 to 14% of another group of patients in the same region had oral KS.89
Oral KS manifests as red to purple macules, papules, or nodules that may ulcerate and cause local tissue destruction (Figure 3).90 The palate and gingivae are the most commonly affected intraoral sites. In HIV disease, the oral cavity is commonly affected and is the first clinical site of KS in 20% of cases, while it occurs concomitantly with skin and visceral involvement in up to 70% of patients.92
At present, there is no preventive vaccine or antiviral agent for AIDS-related KS. Treatment is thus directed towards the elimination or at least reduction of cosmetically unacceptable lesions, the reduction of painful or unsightly edema or lymphadenopathy, as well as the relief of symptoms caused by visceral involvement.93 Local therapy may be effective for limited disease, but systemic therapy is required for disseminated KS. ART is useful in the management of HIV-related KS because it reduces the HIV viral load and raises the CD4+ T cell count, both of which indirectly contribute to the pathogenesis of KS.90 Older approaches for oral KS have included local radiation,93 laser therapy,94 surgical excision, and cytotoxic therapy with vinca alkaloids (vinblastine, vincristine and vinorelbine) and bleomycin.95 However, only five agents are currently approved by the FDA for the treatment of KS: alitretinoin gel for topical therapy and liposomal daunorubicin and oloxorubucin, paclitaxel, and interferon-alpha for systemic therapy.91,96
Human Papillomavirus (HPV)The reported increased prevalence of oral condylomas since the widespread administration of HAART suggests that a drug or combination of drugs used to treat HIV may be a risk factor for oral HPV infection.97 However, HPV infection has taken on much greater significance in recent years because the frequency of HPV infection has increased in groups examined following the introduction of HAART and especially because this virus may be of etiological significance in the development of oral squamous cell carcinoma (OSCC) [reviewed in detail elsewhere].98
HIV-infected individuals may be more likely to carry HPV in the mouth than immunocompetent individuals are (25.3 vs. 7.6%, respectively). They are also more likely to be infected by more than one HPV genotype (5.8 vs. 1.5%) and carry a high-risk genotype, such as HPV-16. Oral warts are uncommon in immunocompetent individuals but oral mucosal abnormalities such as papillomas are more likely to arise in those HPV-infected individuals with concomitant HIV disease.97 Oral HPV infection (both the carriage and/or clinical presentation) seems to be associated with male gender, HSV-2 seropositivity, and older age in HIV-seronegative individuals. In HIV-infected populations, the risk factors for oral HPV infection still include male sex and HSV-2 serostatus, but the strongest association is with oral-genital contact.99
The etiology of the high prevalence of oral HPV infection in HIV-infected individuals is unclear. It is possible that HIV-infected individuals exhibiting high-risk sexual practices frequently acquire oral HPV infection due to multiple exposures simply as a side effect of these high-risk practices. Alternatively, the high rates of HPV detection could be due to increased HPV replication and/or persistence rather than increased HPV acquisition. If persistence of oral HPV leads to HPV-related disease, as it does in the genital tract, increased persistence of HPV could also explain the increased prevalence of oral warts in HAART-treated HIV-positive individuals.100 It is also possible that the individuals who are placed on HAART have already been immunosuppressed long enough to surpass some critical threshold for developing HPV-related disease that cannot be then reversed with therapy.101
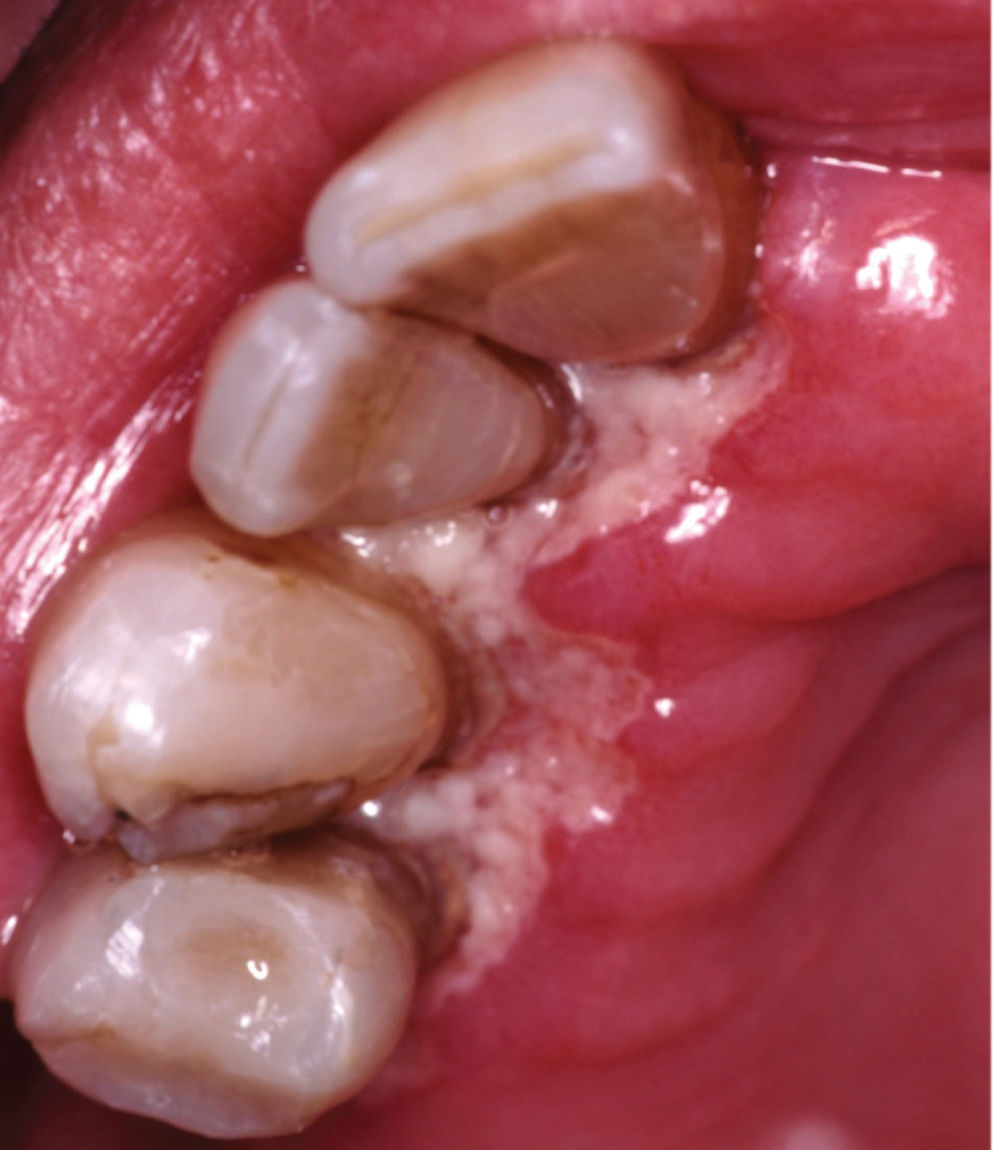
Gingival and Periodontal DiseasesGingivitis and PeriodontitisGingival and periodontal disease is common in HIV infection, particularly in individuals residing in or who have migrated from the developing world. The gingival and periodontal diseases associated with HIV include linear gingival erythema, NUG (Figure 4), NUP, and necrotizing stomatitis.102
Nevertheless, the most common gingival and periodontal features of HIV-infected persons are plaque related to gingivitis and chronic periodontitis, similar to that found in non-HIV-infected individuals. “Chronic” periodontal disease has been described to be more common and/or more aggressive in HIV-infected patients.103
The possible occurrence of HIV-specific periodontal disease has been observed in some but not all groups of HIV-infected patients, suggesting that HIV infection alone does not predispose patients to pocketing, attachment loss, or bleeding on probing.104 The reported prevalence of HIV-related gingival and periodontal disease (excluding opportunistic infections and malignancy) varies from 0 to 47% in adults105 and from 0 to 20% in children; NUG and NUP are less prevalent, varying from 2.2 to 5%.106 Although aspects of HIV-induced immunosuppression have been proposed as the likely cause of HIV-related gingival and periodontal disease, HIV-infected patients often have other relevant risk factors, such as tobacco smoking and poor oral hygiene, and these factors alone can explain the increased prevalence of the disease.107
HIV-related salivary gland diseaseSalivary gland enlargementHIV-associated salivary gland disease (HIV-SGD) is characterized by salivary gland swelling in one or both parotid glands with or without xerostomia.75 In some patients, salivary gland enlargement may be the first clinical manifestation of HIV infection. Inflammatory/infectious conditions are the second most common group of salivary gland disorders in HIV disease, followed by neoplastic lesions.108
Salivary gland enlargement occurs in approximately 3 to 0% of adult patients infected with HIV, with a higher frequency in children, and it may be the first clinical manifestation of HIV. The swelling arises as a consequence of a variety of etiologies, including reactive/inflammatory conditions, infections, and neoplasms.109
Typically, the parotid gland is affected, and the swelling is bilateral, sometimes cystic, and seen in association with generalized lymphadenopathy, persistent circulating CD8+ lymphocytosis and diffuse visceral CD8+ lymphocytic infiltration. This symptom complex is termed diffuse infiltrative (CD8) lymphocytosis syndrome (DILS).110,111
In other cases, the swelling can be caused by benign parotid hypertrophy or cystic benign lymphoepithelial lesions (BLLs). These cysts may originate from an HIV-related reactive lymphoproliferation of glandular epithelium trapped in normal intraparotid lymph nodes remaining from embryologic development or from a ductal obstruction of lymphoid proliferation leading to ductal dilatation.112
Inflammatory or infectious conditions are the second most common group of salivary gland disorders in HIV disease, followed by neoplastic lesions. Indeed, HIV-infected patients have a high incidence of salivary NHL and secondary malignant neoplasms (e.g., Kaposi’s sarcoma) accounting for 10% of malignant salivary gland neoplasms in HIV disease.108
Xerostomia is a common symptom in HIV-infected individuals, and it has many potential causes. For example, it may accompany salivary gland enlargement in DILS,113 and it has also been reported to occur with HAART particularly some of the NRTIs and PIs.114 Antiretroviral agents such as lamivudine, didanosine (DDI) and the protease inhibitors can cause reduced salivary output. In addition, the long-term use of other non-HIV-related medications, such as many classes of antidepressants, can also lead to oral dryness.115
Treatment of salivary gland enlargement in HIV disease remains non-specific. While the frequency of HIV-SGD may increase with HAART, it occasionally resolves with HAART. Superficial parotidectomy has been advocated to alleviate the HIV-associated parotid swelling, but its application may be limited because of associated morbidity. Aspiration of cystic lesions can be of some transient benefit, and injections of tetracycline and doxycycline have minimal success due the presence of multiple cysts.116 Benign parotid hypertrophy in HIV disease can be treated with external radiation therapy (24 Gy in 1.5 Gy daily fractions), with significant improvements in cosmetic control and long-term results.117
XerostomiaIn general dental practice, many patients complain of xerostomia, typically secondary to drug therapy (particularly antidepressants and anxiolytics)118 but sometimes a sign of Sjogren’s syndrome.119 However, if a patient is known to have HIV disease, the xerostomia likely reflects HIV salivary gland disease or is an adverse side effect of ART.120 All patients with xerostomia regardless of HIV serostatus must be given appropriate advice regarding the increased risk of caries (particularly cervical and root caries) and gingival inflammation. In addition, salivary substitutes and sialogogues may lessen the symptoms of xerostomia.121
Neoplastic tumorsNon-Hodgkin’s lymphomaNon-Hodgkin’s lymphoma (NHL) is the second most common HIV-associated tumor. As with KS, the frequency of this tumor has fallen with the introduction of ART; however, it is still a very common tumor of HIV-infected individuals in the developing world.122
A variety of NHLs can arise in the mouth in HIV disease; in fact, a rare type called plasmablastic lymphoma seems to nearly always arise exclusively in the mouth.123 The oral manifestations of NHL include soft tissue masses with or without ulceration and tissue necrosis, usually involving the gingival, palatal, and alveolar mucosa. The optimal treatment for HIV-associated NHL remains unclear. However, oral NHL management primarily entails systemic therapy124 with intensive high-dose chemotherapy and autologous stem cell transplantation; this latter treatment has resulted in sustained complete remissions in selected patients with recurrent chemosensitive disease.125
DENTAL CONSIDERATIONS FOR PEDIATRIC HIV PATIENTSThe oral manifestations of HIV infection in children are generally similar to those in adults, but there are some differences particularly with regards to caries and possibly the eruption times of teeth. HIV-infected children may be more vulnerable to dental caries, affecting both the deciduous and permanent dentition, than healthy subjects. However, the caries in HIV-infected children appear to be largely similar to those in other chronically sick children of comparable age.126
Children with HIV disease may exhibit a different decay pattern than healthy children, possibly related to HIV-associated xerostomia.127,128 Part of the increase in caries in HIV-infected children may be attributed to the high carbohydrate and sugar intake required to prevent or treat any failure to thrive and to the ingestion of sucrose-based medications (particularly antibiotics and antifungals, but also antiretrovirals such as zidovudine.)129
In the developing world, poverty and lack of fluoride supplements may also contribute to an increased risk of dental caries in children with HIV disease.130 Both delayed and accelerated eruption of permanent teeth and over-retention of primary teeth have been observed in some HIV-infected children.130 The accelerated eruption pattern may be related to concurrent or previous dental and periodontal disease, but the exact cause of delayed eruption of teeth is unknown; the poor general health status of some children, particularly when there is malnutrition, may be an important co-factor.72
CONCLUSIONSAn understanding of the immunopathogenesis of HIV infection is a major prerequisite for rationally improving therapeutic strategies and developing immunotherapeutics and prophylactic vaccines. A better understanding of the oral manifestations of HIV/AIDS in both adults and children has implications for all dental health care workers in the world. We must assume that it is almost impossible to recognize if patients have, or are liable to have, HIV infection, and this knowledge must be reflected in the maintenance and continued updating of infection control policies in clinical practice. For that reason, it is necessary to integrate continuous and careful medical care of oral health as a part of the treatment for people with HIV/AIDS. The prevention, diagnosis, treatment, and control of these oral manifestations should be part of the objectives of every dental health professional; these professionals should hereafter also be informed about the relationship between immunological markers and the appearance of oral lesions. Key elements of the response to the HIV/AIDS epidemic include the support of the World Universal Public Health System, the provision of universal access to highly active antiretroviral therapy, and the creation of harm reduction projects that are politically and financially supported by federal governments.