To identify predictors of low cardiac output and mortality in decompensated heart failure.
INTRODUCTIONIntroduction: Patients with decompensated heart failure have a high mortality rate, especially those patients with low cardiac output. However, this clinical presentation is uncommon, and its management is controversial.
METHODSWe studied a cohort of 452 patients hospitalized with decompensated heart failure with an ejection fraction of <0.45. Patients underwent clinical-hemodynamic assessment and Chagas disease immunoenzymatic assay. Low cardiac output was defined according to L and C clinical-hemodynamic profiles. Multivariate analyses assessed clinical outcomes. P<0.05 was considered significant.
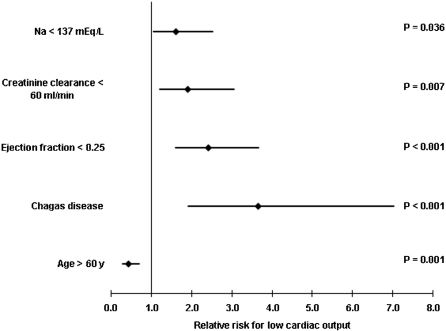
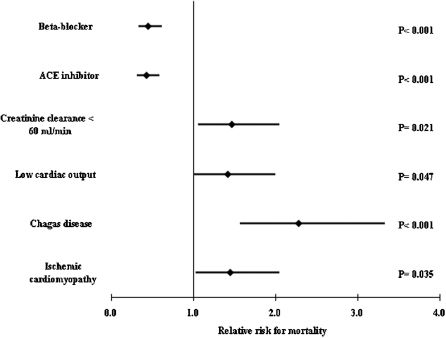
RESULTSThe mean age was 60.1 years; 245 (54.2%) patients were >60 years, and 64.6% were men. Low cardiac output was present in 281 (63%) patients on admission. Chagas disease was the cause of heart failure in 92 (20.4%) patients who had higher B type natriuretic peptide levels (1,978.38 vs. 1,697.64 pg/mL; P = 0.015). Predictors of low cardiac output were Chagas disease (RR: 3.655, P<0.001), lower ejection fraction (RR: 2.414, P<0.001), hyponatremia (RR: 1.618, P = 0.036), and renal dysfunction (RR: 1.916, P = 0.007). Elderly patients were inversely associated with low cardiac output (RR: 0.436, P = 0.001). Predictors of mortality were Chagas disease (RR: 2.286, P<0.001), ischemic etiology (RR: 1.449, P = 0.035), and low cardiac output (RR: 1.419, P = 0.047).
CONCLUSIONSIn severe decompensated heart failure, predictors of low cardiac output are Chagas disease, lower ejection fraction, hyponatremia, and renal dysfunction. Additionally, Chagas disease patients have higher B type natriuretic peptide levels and a worse prognosis independent of lower ejection fraction.
Low cardiac output as the presenting feature of acute decompensated heart failure is uncommon, ranging from 8.9%1 to 9.6%2 of hospitalized patients. Clinical-hemodynamic assessment allows recognition of low cardiac output, based on L and C profiles. In fact, Nohria et al3 demonstrated that the L profile has a cardiac index average of 1.6 L/min/M2, and the C profile has an average of 1.9 L/min/M2. Nevertheless, management of low cardiac output has many difficulties and complexities. It is thought that low cardiac output occurs in more advanced stages and in more depressed systolic ventricular function. In addition, factors such as the presence of hyponatremia and worsening of renal function related to this situation could predict low cardiac output. In our hospital, we have found that patients with Chagas disease have more low cardiac output; however, published heart failure registries have not indicated this association.
Chagas disease is a major health problem in endemic areas, mostly in South America, and its cardiac form causes high mortality. Moreover, Chagas disease could appear in nonendemic countries because of migration.4 Therefore, it is important to describe the clinical presentation of heart failure caused by Chagas disease.
Similarly, little data about decompensated heart failure in the elderly are available, even in heart failure registries. Older patients are more often referred to hospice after hospital discharge; in addition, older women have a greater probability of a longer hospital stay.5 It has been established that elderly patients have more diastolic than systolic dysfunction in heart failure, including that caused by myocardial ischemia. However, clinical presentation and outcomes of elderly patients with heart failure and depressed left ventricular ejection fraction have not been studied.
Therefore, the purpose of this study was to identify predictors of low cardiac output and mortality in decompensated severe heart failure.
METHODS AND MATERIALWe selected a cohort of patients hospitalized for acute decompensation of chronic heart failure between 2005 and 2007, referred to a tertiary cardiology hospital. Inclusion criteria were worsening of dyspnea at rest and ejection fraction <0.45. Exclusion criteria were aortic stenosis, acute coronary syndromes, stroke, and coronary bypass or percutaneous coronary angioplasty in the previous 2 months.
We divided the patients according to age ≥60 years and <60 years, and we analyzed variables related to heart failure. We also divided the patients according to the presence or absence of Chagas disease, defined by serologic testing with enzyme immunoassay.
Low cardiac output was defined based on C (with congestion signs) and L (without congestion signs) profiles in clinical hemodynamic assessment.6 Compromised perfusion was assessed by the presence of a narrow pulse pressure, symptomatic hypotension, cool extremities, impaired mental function, or a combination of these.
Laboratory analyses were done at admission and follow-up, according to clinical criteria. The “Modification of Diet in Renal Disease” (MDRD) method calculated creatinine clearance,7 through the following formula: glomerular filtration rate = 186 × [serum creatinine] -1,154 × [age] -0.203 × [0.742 if patient is female] × [1,212 if patient is black]. After that, we defined 3 groups according to renal function: G1: baseline creatinine clearance >60 mL/min; G2: baseline creatinine clearance <60 mL/min, and increase >10 mL/min during hospitalization (with recovery); and G3: baseline creatinine clearance <60 mL/min, and sustained renal dysfunction during hospitalization (without recovery). Hyponatremia was defined as serum Na <137 mEq/L.
Plasma concentrations of B type natriuretic peptide (BNP) were analyzed by using ADVIA Centaur BNP-analysis (Bayer, Leverkusen, Germany), and expressed in pg/mL.
Heart failure management routineAfter definition of the clinical-hemodynamic profile for each patient, those with low output signs (“cold”, L and C profiles) were treated with intravenous inotropic support: dobutamine, milrinone, or levosimendan. Patients with symptoms and signs of pulmonary or systemic congestion received a diuretic, intravenous furosemide with or without hydrochlorothiazide. Oral vasodilators, such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blocker, and hydralazine, were optimized during hospitalization, to reach the target daily dosage, or in association between these vasodilators. Beta-blockers currently being taken were continued, but their dosages were reduced by half at admission. Patients were followed up after discharge through telephone contact or computerized medical consults.
Statistical analysisContinuous variables are expressed as mean and standard deviation, and were compared by using the Student t test. BNP levels were transformed by logarithmic correction to compare groups. Categorical variables are expressed by number and proportion and compared by using the chi-square test or Fisher's test. We considered P-value <0.05 (2-tailed) as significant.
Predictors of low cardiac output were defined by logistic regression,8 and they are expressed by relative risk and 95% confidence interval. Predictors of all-cause mortality were defined by Cox regression, and they are expressed by relative risk and 95% confidence interval. Survival curves were developed by using the Kaplan-Meier method and compared by using log-rank test.9
Informed consent was obtained at admission, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
RESULTSWe studied 452 patients between February of 2005 and April 2007. As show in Table 1, the majority of patients were men, with a mean age 60.1 years old. According to the cause of heart failure, 151 (33.9%) patients had ischemic myocardiopathy, 107 (24.0%) patients had hypertensive myocardiopathy, and 92 (20.6%) patients had Chagas disease.
Baseline characteristics.
| Men (%) | 292 (64.6) |
| Age (years) | 60.1 ± 14.8 |
| Age > 60 years old (%) | 245 (54.9) |
| LVEF | 0.26 ± 0.08 |
| Intravenous inotropic drug | 281 (63.0) |
| Beta-blocker | 312 (69.9) |
| ACE inhibitor | 277 (62.1) |
| Creatinine clearance (ml/min*1.73 m2) | 57.4 ± 26.9 |
| Sodium (mEq/L) | 137.4 ± 4.4 |
LVEF: Left ventricular ejection fraction, ACE: angiotensin-converting enzyme
Moreover, Chagas disease patients were younger (53.0 ± 12.7 vs. 62.0 ± 14.7 years, P<0.001), and they had lower ejection fraction (22.9 ± 8.0 vs. 26.7 ± 8.4%, P<0.001). In addition, Chagas disease (n = 45) patients had higher BNP levels (1,978.38 vs. 1,697.64 pg/mL; P = 0.015).
On the other hand, patients older than 60 years old had higher ejection fractions, and they (n = 111) had similar BNP levels (1,823.53 vs. 1,683.53 pg/mL, P = 0.375).
Predictors of low cardiac outputLow cardiac output was present in 251 (63%) who required intravenous inotropic drugs, which were dobutamine in 249 patients, levosimendan in 22 patients, and milrinone in 21 patients, and included simultaneous infusion. Variables included in the logistic regression analysis were Chagas disease, ischemic cardiomyopathy, age >60 years, admission creatinine clearance <60 mL/min/M2, ejection fraction <0.25, serum Na <137 mEq/L, and Caucasian ethnicity. Moreover, predictors of low cardiac output, as shown in Figure 1, were Chagas disease, lower ejection fraction, and renal dysfunction. Conversely, old age was inversely associated with inotropic support.
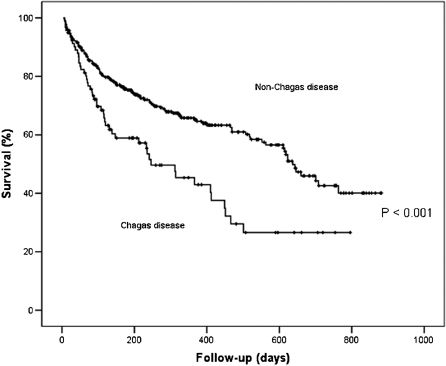
Predictors of all-cause mortalityAt a mean follow-up of 17.2 ± 0.66 months, 173 (38.8%) patients had died. Variables included in Cox regression analysis were Chagas disease, ischemic cardiomyopathy, low cardiac output, age >60 years, admission creatinine clearance <60 mL/min/M2, ejection fraction <0.25, serum Na <137 mEq/L, and ACE inhibitor, beta-blocker, hydralazine, and spironolactone. Multivariate analysis identified Chagas disease, ischemic myocardiopathy, low cardiac output, and renal dysfunction (creatinine clearance <60 mL/min) as predictors of mortality, and ACE inhibitor and beta-blocker as predictors of lower mortality (Figure 2). Survival curves showed a worse prognosis for Chagas disease (Figure 3).
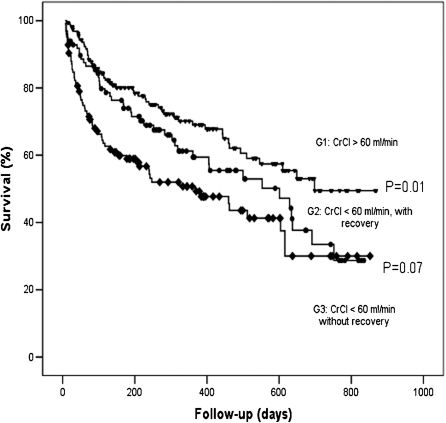
Renal function during hospitalizationTwo or more measurements of creatinine clearance were available in 384 patients. Creatinine clearance was <60 mL/min in 223 patients (58.1%) at admission, and 98 (44%) patients had an increase of 10 mL/min during hospitalization. The remaining patients had first creatinine clearance >60 mL/min, and they kept it above this value during hospitalization. The group with recovery of renal function had a trend of lower mortality than patients with sustained renal dysfunction (Figure 4).
Univariate analysis according to etiology.
| Variables | Chagas disease (N = 92) | Non-Chagas disease (N = 360) | P-value |
|---|---|---|---|
| Age (y) | 53.0 ± 12.7 | 62.0 ± 14.7 | < 0.001 |
| Men (%) | 55 (59.8%) | 237 (65.8%) | 0.279 |
| ACE inhibitor (%) | 59 (64%) | 218 (60.5%) | 0.530 |
| Beta blocker (%) | 62 (67.4%) | 250 (69.4%) | 0.704 |
| Low cardiac output (%) | 79 (85.9%) | 201 (55.8%) | <0.001 |
| Ejection fraction (%) | 22.9 ± 8.0 | 26.7 ± 8.4 | < 0.001 |
| Creatinine clearance (mL/min*M2) | 61.3 ± 23.0 | 56.4 ± 27.7 | 0.119 |
| Serum Na (mEq/L) | 135.8 ± 4.6 | 137.8 ± 4.3 | < 0.001 |
| BNP (pg/mL) (available) | 1,978.38 ± 1,314.02 (N = 45) | 1,697.64 ± 1,376.06 (N = 161) | 0.015 |
ACE: angiotensin-converting enzyme, BNP : B type Natriuretic peptide
To our knowledge, this is the first study to demonstrate an association between Chagas disease heart failure and low cardiac output during decompensation.
The main finding of the present study is that Chagas disease is a predictor of low cardiac output and mortality in decompensated severe heart failure. Additionally, BNP levels were higher in Chagas disease patients. We also observed an association between older age and lower incidence of low cardiac output. Older patients had higher ejection fractions and similar BNP levels compared with younger patients.
The presence of low cardiac output in the setting of decompensated heart failure represents a very high-risk situation. We emphasize that this hemodynamic profile has important differences from large-scale clinical registries such as ADHERE.
In our sample, low cardiac output was a more frequent profile of heart failure decompensation. This finding is compatible with findings in patients referred to tertiary cardiology centers for treatment of advanced heart failure. Although, low cardiac output is uncommon in the general setting, it requires a type of management with complicated and controversial decisions, such as the choice between vasodilators and inotropes.
In this context, clinical assessment of the hemodynamic profile helps us to identify low cardiac output, and consequently adequate therapy can be adopted. L and C profiles show a cardiac index lower (1.6 ± 0.5 and 1.9± 0.7 L/min/m2, respectively) than normal limits6. In addition, predictors of low cardiac output may be helpful.
Chronic Trypanosoma cruzi infection (Chagas disease) frequently causes heart failure in persons living in rural areas of South America; however, it is becoming a problem in nonendemic areas or countries, as has happened in our city, mostly due to the migration of populations.
Differences in hystopathologic characteristics,10 malignant cardiac arrhythmia and higher mortality have been described in Chagas cardiomyopathy.11 In principle, a worse prognosis was attributed to more intense myocardial damage and left ventricular dysfunction. Most trials that have studied Chagas disease have been done in patients with stable chronic heart failure; however, the clinical presentation of decompensated heart failure has not been described.
Chagas cardiomyopathy causes more severe systolic dysfunction, although, in our sample it predicted low cardiac output independently from lower left ventricular ejection fraction. Consequently, it is probable that peripheral vascular resistance increased. Negrão et al12 found a similar muscle sympathetic nerve activity in Chagas cardiomyopathy in relation to other causes of heart failure. In addition, forearm vascular conductance was reduced, as was non-Chagas heart failure. Moreover, lower levels of catecholamine in Chagas heart failure have been demonstrated, differently from non-Chagas disease patients13. It is possible that problems of adaptive mechanisms, such as the lack of sympathetic and renin-angiotensin-system activation, could contribute to low cardiac output like decompensated heart failure presentation. In addition, our Chagas disease patients had higher levels of BNP than non-Chagas heart failure patients had, which is compatible with more severe left ventricular dysfunction. Other authors have described this finding;14 however, baseline BNP of our sample was very high in both Chagas and non-Chagas heart failure.
Others predictors of low cardiac output and mortalityDecompensated heart failure in elderly patients has been studied very little as yet. In our cohort, we found a lower incidence of low cardiac output and similar BNP levels than those in younger patients. These findings are compatible with findings with more frequent ischemic causes and reduced compliance of the great arteries in elderly people.
Decompensated heart failure in the elderly is characterized by more diastolic dysfunction, more worsening renal function,15 worse cognitive function,16 and a worse prognosis. Our results are compatible with those of the ADHERE study, which found younger age and ejection fraction in patients who required inotropic therapy.2
We found a trend of a better prognosis among patients who recovered from renal dysfunction. The pathophysiology of cardio-renal syndrome involves interaction between neurohormonal changes, hemodynamic condition, and drug therapy. In addition, the presence of renal dysfunction in heart failure has been described as a predictor of mortality in compensated as well as decompensated phases. However, recovery of renal function has not been evaluated in the setting of decompensated heart failure.
It is possible that recovery of renal function just reflects a better cardiac situation during decompensation. Nevertheless, renal recovery indicates a better prognosis through widely available MDRD creatinine clearance. It is likely that hemodynamic condition, ie, low cardiac output, is the most important factor of worsening renal function during the decompensation of heart failure.
Because the exponential relationship between glomerular filtration rate and serum creatinine, small changes in serum creatinine result in greater reductions in its clearance at low initial serum creatinine compared with higher serum creatinine levels.17 Despite of this limitation the majority of recently published studies about renal dysfunction in heart failure have used calculated glomerular filtration rate based on MDRD equation.18,19 Additionally, MDRD equation has been validated in heart failure patients.20
Study limitationsOur study assessed the clinical presentation of patients with decompensated heart failure; however, we did not study the neurohormonal condition of these patients. We defined low cardiac output through clinical hemodynamic bedside assessment, but actually we did not measure cardiac index or ventricular filling pressures.
CONCLUSIONSIn severe decompensated heart failure, Chagas disease, lower ejection fraction, hyponatremia, and renal dysfunction predicted low cardiac output. Additionally, Chagas disease patients had greater BNP levels and a worse prognosis, independently from lower ejection fraction.