Prenatal tobacco exposure interferes with neonatal outcomes.
OBJECTIVE:To determine the neonatal neurobehavioral effects of in utero tobacco exposure.
METHODS:This prospective cross-sectional study included healthy, term, with birth weight appropriate for gestacional age neonates without exposure to alcohol, drugs, or infections, born to adolescent mothers without psychiatric disorders or post-traumatic stress. Infants were classified according to in utero tobacco exposure, as identified by the Composite International Diagnostic Interview administered to mothers. Neurobehavior was assessed by the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Both tools were administered between 24 and 72 hours after birth. Neurobehavioral outcomes were compared between exposed and non-exposed infants by ANOVA. The associations between neurobehavioral scores and number of cigarettes smoked were studied by linear correlation.
RESULTS:During the study, 928 newborns of adolescent mothers were born, and 388 were included in the study. Of these, 23 were exposed to tobacco, and 365 neonates were not exposed. There were no differences between the groups in gestational age, birth weight, post-natal age at the exam, or time between last feeding and exam. Exposed neonates showed higher scores on arousal (p = 0.004), excitability (p = 0.003), and stress/abstinence signals (p = 0.019) and a lower score on regulation (p = 0.025). After adjusting for the type of anesthesia, mode of delivery, gender, age at neurologic exam, exam duration and time between last feeding and exam, differences in arousal and excitability remained significant. The mean number of cigarettes consumed daily was positively correlated with lethargy (p = 0.013) and inversely with attention (p = 0.043).
CONCLUSIONS:Neonates exposed in utero to tobacco showed worse neurobehavioral performance between 24 and 48 hours of life.
In 2007, there were 183,440 births to mothers aged 15–19 years in the United States, a birth rate of 42.5 per 1,000 women in this age group.1 In Brazil in 2007, 21% of births occurred in adolescent mothers (10 to 20 years old).2 Adolescents have a high prevalence of substance abuse.3 Despite a significant decline in current smoking frequency since 1996, in 2008, one-fifth of young Americans were smokers by the time they completed high school.4 In a study published in 2010, 14.2% of 1,367 private-school–attending female adolescents from 28 schools in São Paulo, Brazil, reported that they had used tobacco in the past month.5
Tobacco use during pregnancy is a public health problem. Approximately 13% of American women report daily tobacco use during the last three months of pregnancy.6 Bloch et al. examined pregnant women's use of cigarettes and other tobacco products in nine nations in Latin America, Asia, and Africa. A face-to-face survey was administered to 7,961 pregnant women (more than 700 per site) between Oct 2004 and Sept 2005. The highest levels of current smoking were found in Uruguay (18.3%), Argentina (10.3%), and Brazil (6.1%).7 Furthermore, among substances of abuse, tobacco is the one that women are least prone to discontinue during pregnancy.8 Regarding data on pregnancy during adolescence and smoking during pregnancy, a Canadian study of 1,134 women done by Johnson et al. found that smokers during pregnancy were 2.4 times more likely to be under 25 years of age.9 In addition, pregnancy during adolescence is associated with lower socio-economic level, lack of employment, and the presence of a dysfunctional family.10 Therefore, the relationship among smoking, pregnancy, and adolescence is complex and mediated by several contextual factors that are not completely understood.
Maternal smoking during gestation can interfere with fetal growth and development in different ways. Tobacco metabolites are vasoconstrictors, reducing the uterine blood flow and causing fetal intrauterine growth retardation.11 In the central nervous system, nicotine upregulates nicotinic cholinergic receptor binding sites, interfering with synaptic activity,12 leading to fetal neuronal damage and loss. Even low levels of cigarette smoking during gestation can interfere with fetal growth and cerebral development because the fetal nicotine concentration is 15% higher than the maternal concentration.13
Although there are many studies on the effects of maternal smoking during gestation, few of them focus on neonatal neurobehavioral consequences. Using different tools to assess neonatal behavior, these studies show that newborn infants of mothers who use tobacco during pregnancy are excitable and irritable, have more disturbances of tone and abnormal reflexes, need more handling, and are less alert.14–16 Among the different tools used in the literature, the Neonatal Intensive Care Unit Neurobehavior Network Scale (NNNS) was specifically designed to characterize the neonatal effects of prenatal drug exposure. Only one study has been published using the NNNS to assess the neurobehavioral effects of antenatal tobacco exposure in infants during the first days of life. Law et al.17 found that infants exposed to tobacco during gestation were more excitable and hypertonic; they required more handling and showed more stress/abstinence signs than non-exposed neonates in the first 48 hours of life.
This study aimed to determine whether prenatal exposure to tobacco interferes with the neurobehavior of newborn infants of adolescent mothers assessed between 24 and 72 hours of life and to determine whether a dose–response relationship exists between the number of cigarettes smoked during gestation and neurobehavioral assessment.
MATERIALS AND METHODSThis cross-sectional study with prospective data collection was performed in the Maternity Hospital Mario de Moraes Altenfelder Silva, a third-level, city-owned hospital in São Paulo, Brazil, between July 2001 and November 2002. The study was approved by the ethical committees of the hospital and the Federal University of São Paulo and was funded by the State of São Paulo Research Support Foundation (FAPESP – grant number 2000/10.293-5). After birth, one of the investigators explained the study and read the informed consent to each adolescent mother. After resolving possible doubts, the investigator asked her to sign the consent form.
Term neonates were included in the study based on the following criteria: a maternal informed consent form had been signed; the mother was an adolescent aged 10 years to 19 years, 11 months and 29 days; and the infant was a full-term newborn, defined as having a gestational age between 37 weeks and 41 weeks and six days, according to the best obstetric estimate or by the New Ballard method.18 Neonates with conditions or risk factors that could potentially interfere with their neurobehavioral assessment were excluded. The following groups of neonates were excluded: those whose mothers had positive serology for syphilis, toxoplasmosis, cytomegalovirus or human immunodeficiency virus; those whose mothers were diagnosed with maternal depression, anxiety and/or post-traumatic stress disorders during pregnancy; those whose mothers used antidepressant medications during gestation; those whose mothers received opioids, sedatives, and/or anticonvulsants 24 hours prior to delivery or systemic anesthesia during delivery; neonates with antenatal exposure to alcohol, marijuana, cocaine or other illicit drugs; multiple births; neonates with Apgar scores less than 3 in the 1st minute or less than 7 in the 5th minute of life; infants with major congenital malformations or genetic syndromes; those small or large for gestational age; and/or those with any clinical problems, defined by a need for an incubator, oxygen, vascular access, oral tube and/or any medication. Intra-uterine exposure to alcohol, marijuana, cocaine or other illicit drugs was identified by the Composite International Diagnostic Interview (CIDI 2.1).19 Marijuana and cocaine use during gestation were also identified by toxicological analysis of maternal hair and newborn infant meconium samples. A 3-cm segment of hair near the mother's scalp was analyzed by semi-quantitative enzymatic immunoassay after initial decontamination. All positive results were confirmed by gas chromatography and mass spectrometry. Meconium samples were collected in the first 48 h and analyzed by a homogeneous semi-quantitative enzymatic immunoassay.
Neonates and their mothers were studied by a team of five neonatologists, three psychologists and two psychiatrists, and the following steps were performed: 1) Maternal interview by the neonatologists to collect data related to the socio-demographic and obstetrical characteristics of the mothers; 2) administration of CIDI 2.1 to the mothers by the psychologists; 3) clinical examination of the neonate to collect data related to the birth and clinical course until inclusion in the study; 4) neurobehavioral assessment of the infants by the neonatologists with NNNS;20 5) collection of maternal hair and neonatal meconium samples for analysis regarding the presence of marijuana and cocaine metabolites.
CIDI is a standardized and structured interview that identifies psychiatric disorders according to the International Classification of Diseases (CID-10) and the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders as follows: depression; anxiety; post-traumatic stress disorder (PTSD); mania; bipolar disorder; psychotic, dissociative, somatoform or eating disorders; and tobacco, alcohol, or illicit drug use. Tobacco use during gestation was classified as harmful tobacco use (a pattern of psychoactive drug use that causes damage to health, either mental or physical) or as tobacco dependence syndrome (a cluster of physiological, behavioral and cognitive phenomena that indicate the use of a substance takes on a much higher priority for a given individual than other behaviors that once had greater value).21
NNNS evaluates neurological integrity, behavioral function, and the presence of stress and abstinence signs in newborn infants. The NNNS was administered after 24 hours of life, when the global stress response to the birth process was already attenuated, and before 72 hours of life. The exam was carried out in a specific warm, calm room free of intense light by one of four neonatologists previously trained in its procedures. The principal investigator was certified to perform the NNNS at Rhode Island Hospital, Brown University, RI. The other three were trained by the principal investigator. All investigators were masked to tobacco exposure status when performing NNNS. After the complete NNNS evaluation, the items assessed were grouped into 13 variables as described by Boukydis et al.:22 habituation, attention, arousal, regulation, handling, quality of movement, excitability, lethargy, non-optimal reflexes, asymmetry, hypertonicity, hypotonicity, and stress/abstinence signals. Normative data for healthy term infants in the first three days of life were obtained from Tronick et al. (2004).23
STATISTICAL ANALYSESMaternal and neonatal characteristics and NNNS scores were compared between tobacco-exposed and non-exposed neonates using the chi-square or Student's t test. The associations of independent factors with the mean scores of the NNNS variables were tested by analysis of variance (ANOVA). The independent variables analyzed were tobacco exposure (absent vs. present), anesthesia (absent or local vs. regional), type of delivery (vaginal vs. cesarean section), gender (female vs. male), age of the neonate at neurobehavioral exam (≤33 or >33 hours), time between last feeding and neurologic exam (≤21 or >21 minutes), and exam duration (≤25 or >25 minutes). For the last three variables, cutoffs were chosen based on the median value obtained for the whole studied population. Relationships between maternal cigarette consumption during gestation and neurobehavioral scores were assessed by linear regression analysis.
The sample size was determined according to the need to include 15 neonates for each independent variable analyzed in the regression models. Each of the 13 NNNS variables was considered a dependent variable. At least 11 independent categorical variables and five continuous independent variables were analyzed. Therefore, the minimum sample size was calculated as 240. All statistical procedures were performed using Statistical Package for Social Sciences (SPSS) version 10.0 (SPSS Inc., Chicago, IL).
RESULTSFrom July 2001 to November 2002, 3,685 infants were born in the study hospital, and 928 (25%) of them had teenage mothers. Among these, 131 were preterm, five were post-term, and 404 met one or more exclusion criteria: positive serology for congenital infections (26); maternal use of opioids/sedatives 24 hours prior to delivery (7); maternal use of alcohol (except occasional use) or any marijuana and/or cocaine use (52); maternal depression, anxiety and/or post-traumatic stress disorder (166); multiple gestations (6); Apgar score less than 3 at 1 minute or less than 7 at 5 minutes (16); major congenital malformations or genetic syndromes (2); small or large for gestational age (203); and clinical problems (65).
Therefore, 388 infants were studied: 23 infants with antenatal exposure to tobacco and 365 who had not been exposed. Among smoking pregnant women, five had tobacco dependence syndrome, and 18 were harmful tobacco users. They smoked an average of 14±9 cigarettes per day during pregnancy, varying from 2 to 40. Characteristics of the mothers who smoked and those who did not smoke during gestation are displayed in Table 1. Mothers who smoked during gestation had had fewer years of schooling (p = 0.001) and more prenatal care visits (p = 0.020). Both groups were similar regarding maternal age, race, marital status, socioeconomic level, per capita income, delivery mode, and anesthesia type.
Demographic characteristics of the adolescent mothers.
| Exposure to Tobacco during gestation | |||
|---|---|---|---|
| Absent n = 365 | Present n = 23 | p-value | |
| Maternal age | 17.0±1.5 | 17.3±1.4 | 0.284 |
| 10 to 14 years | 22 (6%) | 1 (4%) | 1.000 |
| 15 to 19 years | 343 (94%) | 22 (96%) | |
| White race | 179 (49%) | 7 (30%) | 0.090 |
| Married | 230 (63%) | 33 (61%) | 0.827 |
| Years in school | 7.1±2.2 | 5.6±2.2 | 0.001 |
| Low socioeconomic level | 327/350 (93%) | 22/23 (96%) | 1.000 |
| Per capita income (US$ per month) | 194±133 | 141±87 | 0.064 |
| Prenatal care | |||
| Present | 352 (96%) | 21 (91%) | 0.221 |
| Number of visits | 6.7±2.7 | 8.23±4.8 | 0.020 |
| Vaginal delivery | 265 (73%) | 15 (65%) | 0.474 |
| Regional anesthesia | 278 (76%) | 17 (74%) | 0.803 |
Neonatal characteristics are shown in Table 2. The neonates of the two groups were similar in gestational age, gender, birth weight, length, head circumference, 1st- and 5th-minute Apgar scores, post-natal age at the neurologic exam, time between last feeding and the neurologic exam and length of hospital stay. The neurologic exam of infants exposed to tobacco during gestation lasted longer than of those not exposed (25.7±7.5 vs. 22.4±5.2 minutes; p = 0.004).
Neonatal demographic characteristics and neonatal data at the time of NNNS assessment.
| Exposure to Tobacco during gestation | |||
|---|---|---|---|
| Absent (n = 365) | Present (n = 23) | p-value | |
| Gestational age (weeks) | 0.588 | ||
| Mean±sd | 39.4±1.1 | 39.5±1.0 | |
| Median (minimum–maximum) | 39.4 (37.0–41.9) | 39.4 (37.1–41.6) | |
| Birthweight (g) | 0.704 | ||
| Mean±sd | 3237±292 | 3261±308 | |
| Median (minimum–maximum) | 3225 (2600–4010) | 3245 (2550–3770) | |
| Length (cm) | 48.9±1.5 | 49.4±1.4 | 0.109 |
| Head circumference (cm) | 34.4±1.1 | 34.4±1.1 | 0.948 |
| Male | 193 (53%) | 16 (70%) | 0.135 |
| Apgar at 1 minute | 0.817 | ||
| Mean±sd | 8.1±1.3 | 8.1±1.2 | |
| Median (minimum–maximum) | 9 (3–10) | 8 (4–9) | |
| Apgar at 5 minutes | 0.506 | ||
| Mean±sd | 9.6±0.6 | 9.5±0.6 | |
| Median (minimum–maximum) | 10 (8–10) | 10 (8–10) | |
| Post-natal age (hours) | 0.660 | ||
| Mean±sd | 33.3±6.9 | 33.9±6.9 | |
| Median (minimum–maximum) | 32.4 (24–51.3) | 34.5 (24.2–46) | |
| Exam duration (minutes) | 0.004 | ||
| Mean±sd | 22.4±5.2 | 25.7±7.5 | |
| Median (minimum–maximum) | 20 (10–43) | 25 (10–45) | |
| Time after feeding (minutes) | 0.287 | ||
| Mean±sd | 49.0±54.7 | 36.5±46.2 | |
| Median (minimum–maximum) | 30 (2–300) | 15 (5–150) | |
| Hospital stay (days) | 2.5±0.9 | 2.4±0.5 | 0.900 |
The neurobehavioral scores of the two studied groups are shown in Table 3. Infants exposed to tobacco had higher scores on arousal (4.13±0.78 vs. 3.69±0.69; p = 0.004), excitability (3.83±2.19 vs. 2.64±1.83; p = 0.003), and stress and/or abstinence signals (0.09±0.06 vs. 0.06±0.05; p = 0.019) and lower scores on regulation (5.67±1.01 vs. 6.05±0.77; p = 0.025). No differences were observed in the other NNNS items: habituation, attention, handling, quality of movements, lethargy, non-optimal reflexes, asymmetry, hypertonicity, and hypotonicity. When adjusted for type of anesthesia, delivery mode, gender, age at the neurologic exam, exam duration, and time between the last feeding and the exam, differences in arousal and excitability remained significant (Table 3).
NNNS scores in the studied population.
| Exposure to Tobacco during gestation | ||||
|---|---|---|---|---|
| Absent n = 365 | Present n = 23 | p-value | p-value∗ | |
| Habituation | 6.90±1.44 (n = 222) | 6.72±1.73 (n = 12) | 0.686 | 0.784 |
| Attention | 5.75±1.32 (n = 337) | 5.50±1.35 (n = 20) | 0.417 | 0.462 |
| Arousal | 3.69±0.69 | 4.13±0.78 | 0.004 | 0.031 |
| Regulation | 6.05±0.77 | 5.67±1.01 | 0.025 | 0.085 |
| Handling | 0.35±0.26 | 0.41±0.26 | 0.290 | 0.637 |
| Quality of movement | 5.25±0.45 | 5.15±0.54 | 0.280 | 0.460 |
| Excitability | 2.64±1.83 | 3.83±2.19 | 0.003 | 0.037 |
| Lethargy | 4.60±2.72 | 4.09±2.35 | 0.376 | 0.389 |
| Non-optimal reflexes | 9.27±1.34 | 9.17±1.39 | 0.728 | 0.597 |
| Asymmetry | 0.75±1.10 | 0.48±0.79 | 0.239 | 0.132 |
| Hypertonicity | 0.18±0.38 | 0.26±0.45 | 0.321 | 0.307 |
| Hypotonicity | 0.11±0.35 | 0.22±0.42 | 0.154 | 0.139 |
| Stress/abstinence signals | 0.06±0.05 | 0.09±0.06 | 0.019 | 0.055 |
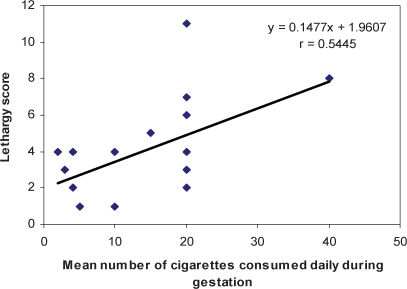
Linear regression analysis showed that the number of cigarettes smoked during gestation was positively correlated with the lethargy score (Score = 0,148 × number of cigarettes + 1.961; r = 0.545; p = 0.013) and inversely correlated with the attention score (Score = −0.069 × number of cigarettes + 6.574; r = 0.496; p = 0.043) (Figures 1 and 2).
Because 21% of the births in Brazil occur in adolescents,2 a group with frequent tobacco use, it is important to know the effects of prenatal tobacco exposure on the neurobehavior of newborn infants of adolescent mothers. In the present study, we administered the NNNS on the second or third day of life to evaluate the neurobehavior of infants of adolescent mothers who smoked tobacco during gestation. Compared to non-exposed infants, infants exposed to tobacco aroused easily, were more excitable, had poorer self-control, and had more stress and/or abstinence signals. After controlling for possible confounders, arousal and excitability scores remained significantly different from the controls. Not only were these items associated with antenatal tobacco exposure, but attention and lethargy scores were also correlated with the number of cigarettes smoked during pregnancy, with a decrease in neurologic performance as the number of cigarettes smoked during pregnancy increased.
Other studies that have used different neurobehavioral exams have shown similar results. Newborn infants exposed to tobacco during gestation and evaluated by the Neonatal Behavioral Assessment Scale (NBAS) between days two and six of life had worse performance on attention tasks, were more difficult to comfort and showed weaker autonomic regulation compared with non-exposed infants.24,25 In another study, Fried et al.14 evaluated infants at 30 days of life and noted associations between prenatal tobacco exposure and hypertonicity and excitability using the Prechtl scale. Oyemade et al.26 administered the NBAS to infants exposed in utero to tobacco and observed worse performance on orientation tasks. On the other hand, some studies did not find neurobehavioral alterations in infants exposed to tobacco during pregnancy.27–29 Many of these studies evaluated tobacco effects as covariates or analyzed tobacco exposure together with other drugs, such as cocaine, alcohol, caffeine, and/or marijuana.
Using the NNNS to evaluate the neurobehavior of infants exposed to tobacco during gestation, Law et al.17 found that these infants were more excitable and hypertonic, required more handling and showed more stress/abstinence signs in the first 48 hours of life. They also showed relationships between higher maternal salivary cotinine level and stress and/or abstinence signals and excitability scores. The number of cigarettes smoked per day during pregnancy was also correlated with neonatal stress and abstinence signs. Stroud et al.30 evaluated term infants exposed in utero to tobacco, aged 10 to 27 days, with the NNNS. Compared to non-exposed infants, the exposed infants had a greater need for handling, worse self-regulation, and a tendency to show greater excitability and arousal. The authors noted that these results suggest that the behavioral effects of nicotine are not only due to a withdrawal process but a persistent deregulation, indicating early vulnerability to later neurobehavioral deficits. This hypothesis is supported by the fact that the half-life of nicotine in newborn infants is approximately 9–11 hours, and the patterns of maternal smoking effects during pregnancy on infant neurobehavior at 10 to 27 days were different from those at one to two days of life, when infants showed stress/abstinence signals and increased muscle tension.30 Yolton et al.31 assessed infants exposed to tobacco in utero at five weeks after birth with the NNNS. After controlling for possible confounders, a higher maternal cotinine level was associated with increased arousal and excitability and decreased self-regulation in white infants. In contrast, among black infants, a higher cotinine level was associated with decreased arousal and excitability and increased self-regulation and hypotonicity. These effects may reflect racial differences in nicotine metabolism. Our study reinforces the previous results that newborn infants antenatally exposed to tobacco arouse easily, are more excitable, and have worse self-regulation and more stress and abstinence signals.
After adjustment for covariates, tobacco-exposed infants showed higher arousal and excitability scores, suggesting that antenatal tobacco exposure may have neurotoxic effects. Nicotine acts on neurotransmitter receptors in the fetal brain, interfering with cell proliferation and differentiation and with synaptic activity.12 In one study, newborn infants of adolescent mothers, assessed between two and five days of life by the Neonatal Brazelton Scale, habituated easier but were less alert and had poorer performance on orientation tasks compared with infants of non-adolescent mothers.32 The combination of an excitable and easily aroused infant and an adolescent mother could interfere with mother–infant bonding during a critical period of development, leading to long-term behavioral deficits.12,33–35
In the present study, mothers smoked an average of 14.1 cigarettes per day, more than reported by Law et al.17 (12.9 cigarettes before pregnancy recognition, 7.5 in the first trimester, 3.8 in the second, and 2.8 in the third). They found that the mother's salivary cotinine level was associated with a higher frequency of stress/abstinence signs and increased excitability scores. In our study, the number of cigarettes consumed during gestation was related to lower attention and higher lethargy scores. We did not verify the number of cigarettes consumed during the different trimesters of gestation, and we did not measure cotinine levels, but salivary cotinine level only reflects tobacco use within the last two days.36 Because the infants in our study were evaluated between 24 and 72 hours of life, it is unlikely that the tobacco effects on newborn neurobehavior were related to passive inhalation, as smoking is not allowed in the hospital.
The large number of exclusion criteria and the adjustment for confounders in the statistical analysis are among the strengths of our study, which allowed us to verify the specific effects of tobacco use during pregnancy on neonatal neurobehavior. At the same time and for the same reason, tobacco-exposed infants represented only 6% of our unbalanced sample, which may be a limitation of the study. A post-hoc analysis to verify the power to detect differences between exposed and non-exposed infants in the variables that remained significant after adjustment for confounders (arousal and excitability) was performed. We found that the power of the study was greater than 90% for the differences and standard deviations observed, with an alpha error of 5%. Moreover, for all studied variables, 18 exposed and non-exposed infants would be required to detect a difference of 1 point in the score, with a standard deviation of 0.8, a power of 80% and an alpha error of 5%.
The cross-sectional design of this study is another limitation. It would be interesting to longitudinally follow the neurobehavioral skills of newborn infants of adolescent mothers exposed and not exposed in utero to tobacco. A single neurobehavioral evaluation should only be used as a screening tool to identify neurobehavioral problems in these groups of newborns.
CONCLUSIONNeonates exposed to tobacco during pregnancy showed worse neurobehavioral performance between 24 and 48 hours of life. Our findings raise concerns about the interaction between families and infants exposed in utero to tobacco, regarding their neurodevelopment. These data reinforce the importance of prenatal care and should encourage pregnant women to reduce or quit tobacco consumption during gestation.
We are grateful to the staff of the Maternity Hospital Mario de Moraes Altenfelder Silva for their help during the data collection.
FUNDING:
This research was fully funded as a thematic project by FAPESP (State of São Paulo Research Support Foundation), grant number 2000/10293-5.